Anti-angiogenic therapies for advanced esophago-gastric cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(04): 253-262
DOI: DOI: 10.4103/0971-5851.144985
Abstract
Neo-vascularization is a vital process for tumor growth and development which involves the interaction between tumor cells and stromal endothelial cells through several growth factors and membranous receptors which ultimately activate pro-angiogenic intracellular signaling pathways. Inhibition of angiogenesis has become a standard treatment option for several tumor types including colorectal cancer, glioblastoma and ovarian cancer. In gastric cancer, the therapeutic role of anti-angiogenic agents is more controversial. Bevacizumab and ramucirumab, two monoclonal antibodies, which target vascular endothelial growth factor-A and vascular endothelial growth factor receptor-2, respectively, have been demonstrated antitumor activity in patients with tumors of the stomach or esophagogastric junction. However, especially for bevacizumab, this antitumor activity has not consistently translated into a survival advantage over standard treatment in randomized trials. In this article, we provide an overview of the role of angiogenesis in gastric cancer and discuss the results of clinical trials that investigated safety and effectiveness of antiangiogenic therapies in this disease. A review of the literature has been done using PubMed, ClinicalTrials.gov website and the ASCO Annual Meeting Library.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Neo-vascularization is a vital process for tumor growth and development which involves the interaction between tumor cells and stromal endothelial cells through several growth factors and membranous receptors which ultimately activate pro-angiogenic intracellular signaling pathways. Inhibition of angiogenesis has become a standard treatment option for several tumor types including colorectal cancer, glioblastoma and ovarian cancer. In gastric cancer, the therapeutic role of anti-angiogenic agents is more controversial. Bevacizumab and ramucirumab, two monoclonal antibodies, which target vascular endothelial growth factor-A and vascular endothelial growth factor receptor-2, respectively, have been demonstrated antitumor activity in patients with tumors of the stomach or esophagogastric junction. However, especially for bevacizumab, this antitumor activity has not consistently translated into a survival advantage over standard treatment in randomized trials. In this article, we provide an overview of the role of angiogenesis in gastric cancer and discuss the results of clinical trials that investigated safety and effectiveness of antiangiogenic therapies in this disease. A review of the literature has been done using PubMed, ClinicalTrials.gov website and the ASCO Annual Meeting Library.
INTRODUCTION
Gastric cancer is the fifth most common type of cancer and the third leading cause of cancer mortality worldwide, with >950,000 new cases and >720,000 deaths estimated in 2012.[1] Despite the improvements achieved with the routine use of peri-operative treatments and the optimization of surgery, in Western countries tumor recurrence occurs in >50% of patients with initially localized disease.[2,3] Furthermore, metastases are present in approximately 50% of cases at diagnosis, and the median survival in this circumstance remains poor, only a minority of patients being alive at 1-year.[4,5] Better survival figures have been reported in Eastern countries, possibly reflecting geographical differences for this malignancy with respect to epidemiology, biology and pharmacogenomics.[6,7]
In recent years, the addition of trastuzumab to standard chemotherapy in patients with HER-2 positive tumors and the increasing use of second-line therapies have led to an improved survival in selected patients.[8,9,10,11] However, a better knowledge of the driving mechanisms of tumor progression and the identification of alternative therapeutic targets are vital.
Historically, angiogenesis has been attributed a crucial role in mediating physiologic processes, including embryogenesis and wound healing.[12] In 1977, Ausprunk and Folkman described for the first time the mechanism of sprouting angiogenesis in tumors.[13] They proposed a multi-step process, including the degradation of the basement membrane of a peri-tumoral capillary, the migration of endothelial cells into the connective tissue, the formation of a solid cord and the conversion of this into an empty capillary. This mechanism, which ultimately ensures blood supply to the tumor has been demonstrated to be vital to sustain tumor growth beyond 2-3 mm[14] and is largely mediated by the hypoxia-inducible factor (HIF)-1α which promotes transcription of pro-angiogenic genes, including the vascular endothelial growth factor (VEGF) gene, under hypoxic conditions.[15]
Vascular endothelial growth factor was isolated for the first time in 1989 as a diffusible heparin-binding polypeptide which specifically targets vascular endothelial cells.[16,17] Subsequently, other VEGF - related genes, including VEGF-B, VEGF-C, VEGF-D, placenta growth factor (PlGF) and platelet-derived endothelial growth factor were found to be associated with the regulation of tumor angiogenesis by encoding growth factors which interact with a number of membranous tyrosine-kinases receptors such as VEGFR-1, VEGFR-2, VEGFR-3 and neuropilin-1 and -2 (NRP-1, NRP-2).[18]
Given the importance of angiogenesis in the mechanisms of tumor growth, proliferation and metastasis, targeting pro-angiogenic signaling pathways has progressively emerged as a rational therapeutic approach for several malignancies.[19] In addition to the antitumoural effects mediated by the direct inhibition of the process of new vessel formation, antiangiogenic therapies have also been associated with indirect antiproliferative effects deriving from the normalization of the disorganized tumor vasculature, which favors intra-tumor delivery of cytotoxic drugs.[20] Whilst the former effect seems to be prerogative of tumors with a poor stroma, the latter effect has been largely described in tumors which are surrounded by a developed stroma.[21] However, despite the compelling biological rationale underlying the use of antiangiogenic therapeutic strategies, a wide range of results has been observed across different tumor types, suggesting a nonunivocal tumor addiction to angiogenesis.[22]
In this article, we discuss the role of angiogenesis in esophago-gastric cancer and review the results of clinical trials with bevacizumab and ramucirumab in the advanced setting.
THE ROLE OF VEGF IN OESOPHAGO-GASTRIC CANCER
In tumors of the gastrointestinal tract, the identification of VEGF as a pro-angiogenic factor expressed by malignant epithelial cells dates back more than two decades. In initial studies of immunohistochemistry and in situ hybridization, VEGF was found to be expressed, especially in areas of tumor necrosis while its receptors, Flt-1 (VEGFR-1) and KDR (VEGFR-2), were localized on the surface of peritumoural stromal endothelial cells.[23] Since then, several studies have investigated the clinical and prognostic relevance of VEGF in oesophagogastric cancers.[24,25,26,27,28,29] In most cases, the association was found between VEGF expression and tumor vascular density and VEGF progressively emerged as a prognostic factor being associated with unfavorable clinico-pathologic features, hematogenous metastases and poor outcome. Interestingly, the role of VEGF as a mediator of angiogenesis in this disease appeared to be more relevant in tumors with an intestinal-type rather than a diffuse-type histological architecture.[29]
The importance of angiogenesis in the pathogenesis and progression of esophagogastric cancers has been confirmed by more recent studies investigating the prognostic role of circulating VEGF. Plasma levels of VEGF have been found to be significantly higher in esophagogastric cancer patients compared to healthy controls and in several series of patients undergoing surgical resection, high levels of VEGF have been reported to be an independent predictor of poor outcome.[30,31,32] Several studies have also investigated the clinical significance of single nucleotide polymorphisms of the VEGF gene. Although in most cases the correlation between some polymorphic variants at specific loci and pattern of tumor relapse or prognosis has been found, the results of these studies have been inconsistent and do not seem to support the hypothesis that genotyping of VEGF could be of clinical relevance.[33,34,35,36]
The main mechanism which leads to transcription of the VEGF gene is the activation of HIF-1α.[15] In the presence of nonhypoxic conditions, HIF-1α undergoes ubiquitination and degradation by proteasomes. Under hypoxic conditions, HIF-1α serves as a transcription factor, which targets several genes, including VEGF and promotes an adaptive angiogenic response to hypoxia.[37] Several reports have shown an association between HIF-1α expression and tumor prognosis in esophagogastric cancer.[38,39,40,41,42] In an elegant preclinical study, Stoeltzing et al. demonstrated that inhibition of the HIF-1α-VEGF axis may have an important therapeutic potential in this disease.[43] In particular, they showed that inactivation of the transcription activity of HIF-1α resulted in a significantly reduced production of VEGF in gastric cancer cells and inhibition of angiogenesis and tumour growth in animal models.
Despite the central role of VEGF in the angiogenic process, several other pro-angiogenic factors have been demonstrated to be actively involved in the mechanisms of angiogenesis, tumor growth, progression and metastasis.[18] In particular, several retrospective studies have reported a strong association between expression of VEGF-C in malignant epithelial cells (and its receptor VEGFR-3 in stromal lymphatic vessels) and lymphangiogenesis.[44,45,46,47,48,49] Altogether, these data seem to suggest a potential differential pattern of tumor progression in esophagogastric cancers, through lymph nodal metastases in tumors with predominant expression of VEGF-C and through hematogeneous metastases in tumors with a predominant expression of VEGF-A.
BEVACIZUMAB IN ADVANCED OESOPHAGOGASTRIC CANCER
Bevacizumab is a monoclonal antibody which exerts an antiangiogenic activity by binding VEGF-A and inhibiting its interaction with VEGFR-1 and VEGFR-2.[50] Preclinical data indicated that this targeted agent had the potential to inhibit tumor neovascularization, tumor vessel density and tumor growth either as monotherapy or in combination with cytotoxic agents.[51] The activity of bevacizumab was subsequently confirmed in a clinical setting and this antiangiogenic agent is now an established treatment option in several malignancies including colorectal cancer, nonsmall cell lung cancer, renal cell carcinoma, glioblastoma and ovarian cancer.[52,53,54,55,56]
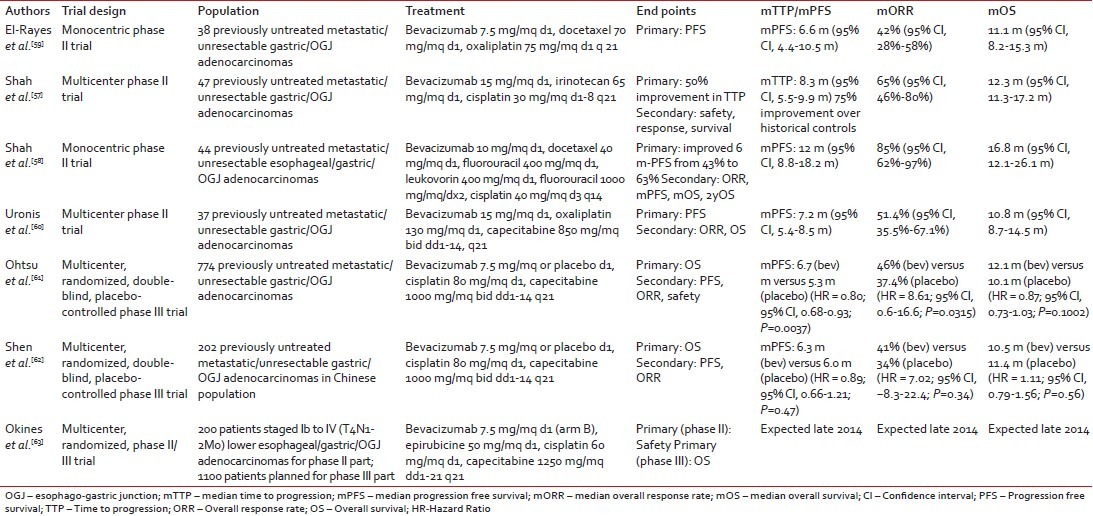
Bevacizumab has also been largely investigated in combination with different chemotherapy regimens in esophagogastric cancer with four completed phase II and two completed phase III clinical trials [Table 1].[57,58,59,60,61,62]
Table 1
Phase II/III clinical trials of bevacizumab in gastric cancer
|
In 2006, Shah et al. reported on the safety and efficacy results of a combination with bevacizumab and cisplatin-irinotecan in patients with locally advanced or metastatic gastric or esophago-gastric junctional (OGJ) adenocarcinoma.[57] In this multicentre phase II trial (n = 47) promising outcome measures were observed with a median time to progression of 8.3 months, the objective response rate of 65% and a median overall survival (OS) of 12.3 months. Moreover, the rate of grade ≥3 adverse events was acceptable with no major safety signals. Few years later, in another small phase II trial conducted in the metastatic setting (n = 44), Shah et al. reported even better results combining bevacizumab with a modified schedule of docetaxel, cisplatin and fluorouracil.[58] Response rate in this patient population was 67% and median progression-free survival (PFS) and OS were 12 months and 16.8 months, respectively. Treatment appeared to be well-tolerated overall with no evidence of increased chemotherapy-relayed toxicities with the addition of bevacizumab. However, it is worth noting that 39% of patients in this trial experienced venous thromboembolism. Less promising results were reported by El-Rayes et al. and Uronis et al. in two smaller phase II trials where bevacizumab was administered in combination with oxaliplatin and docetaxel (RR: 42%, PFS: 6.6 months, OS: 11.1 months) and capecitabine and oxaliplatin (RR: 51%, PFS: 7.2 months, OS: 10.8 months), respectively.[59,60]
The Avastin for Advanced Gastric Cancer trial (AVAGAST) was an international, randomized, double-blind, placebo-controlled phase III trial of bevacizumab in combination with cisplatin and capecitabine in previously untreated unresectable locally advanced or metastatic adenocarcinomas of the stomach or OGJ.[61] The primary endpoint was OS, and the study was powered to demonstrate a 22% reduction in the risk of death (HR = 0.78) with the addition of bevacizumab to standard therapy. It is interesting to note that the dose of bevacizumab used in this trial (2.5 mg/kg/week) was lower than that used in the phase II studies by Shah et al. and Uronis et al. (5 mg/kg/week).[57,58,60]
The study included 774 patients (49% from the Asian-Pacific region, 32% from Europe and 19% from the Americas), of whom only 4% had locally advanced disease. The primary site of the tumor was the OGJ in 13% of patients and liver metastases were present at study entry in 33% of cases. Although a difference in median OS (mOS) in favor of the bevacizumab arm was observed (12.1 vs. 10.1 months), this did not meet the specified criteria for statistical significance (HR = 0.87, 95% CI, 0.73-1.03, P = 0.1002). However, it is worth noting that patients allocated to the investigational arm had a statistically significant better PFS (6.7 months vs. 5.3 months; HR = 0.80; P = 0.0037), RR (46% vs. 37.4%, P = 0.0315) and 1-year survival (50.2% vs. 42.3%, P = 0.0301). More interestingly, preplanned subgroup analyses showed that the beneficial effect of bevacizumab on all study outcome measures was substantially higher among patients recruited in the Americas as opposed to patients recruited in Europe (intermediate effect) or in Asia-Pacific regions (no or limited effect). It is not known whether this regional difference is the result of differences in tumor biology or which may affect bevacizumab activity, or is rather influenced by the imbalance in the use of subsequent treatments (63% in Asian-Pacific regions, 26% in Europe and 19% in the Americas) or the relatively small number of the patients in each subgroup.
Useful data to interpret these results have been subsequently provided by the AVATAR trial. This was a smaller bridging phase III study, which mimicked the design of the AVAGAST trial and was conducted in 202 Chinese patients.[62] Although both baseline patient clinical characteristics and use of poststudy treatments were more similar to the European-American subgroup rather than the Asian-Pacific subgroup of the AVAGAST trial, no significant differences between chemotherapy plus bevacizumab versus chemotherapy alone were reported for any of the outcome measures (OS: 10.5 months vs. 11.4 months, HR = 1.11, P = 0.56; PFS: 6.3 months vs. 6.0 months, HR = 0.89, P = 0.47; RR: 41% vs. 34%, P = 0.35).
In line with other tumor types, bevacizumab has been demonstrated to have an acceptable safety profile in advanced gastric cancer. In both the AVAGAST and AVATAR trial, the incidence of grade ≥3 adverse events was similar between the treatment arms.[61,62] Among chemotherapy-related toxicities, only diarrhea (8% vs. 4%) and hand-foot syndrome (6% vs. 3%) in the AVAGAST trial, and vomiting (22% vs. 10%) and decreased appetite (5% vs. 1%) in the AVATAR trial appeared to be increased with the use of bevacizumab. Interestingly, in these trials no increased incidence of grade ≥3 adverse events of special interest for bevacizumab were observed in the investigational arm compared to the placebo arm with the exception of hypertension in the AVAGAST trial (6% vs. <1>
Based on the negative results of these phase III trials, bevacizumab is currently not an option for gastric cancer patients with unresectable or metastatic tumors. A multicenter randomized phase II/III trial conducted in the UK and sponsored by the Medical Research Council is currently investigating the safety and efficacy of bevacizumab when given in combination with peri-operative ECX chemotherapy in patients with localised oesophago-gastric adenocarcinoma (ST03).[63] The results of this trial are expected in the coming months and despite the different setting of disease, they will certainly provide additional information on the therapeutic potential of bevacizumab in gastric cancer.
RAMUCIRUMAB IN ADVANCED OESOPHAGOGASTRIC CANCER
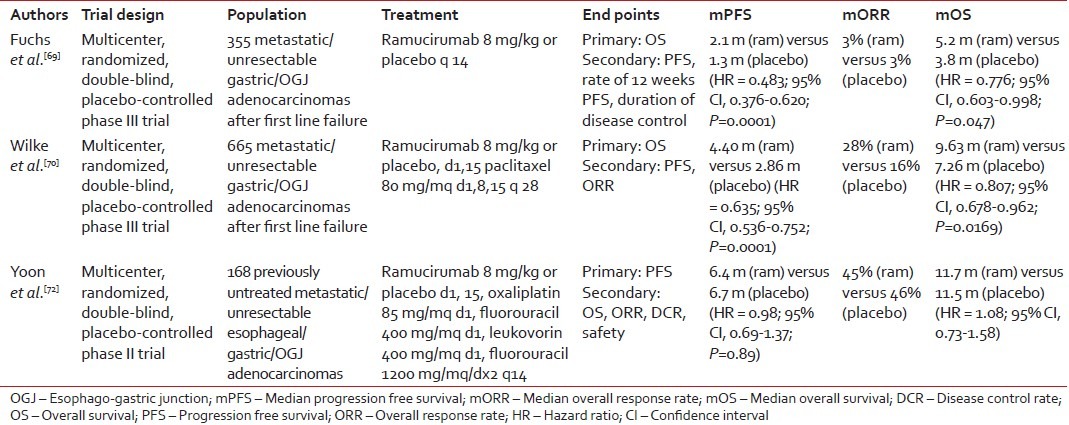
Ramucirumab is a fully humanized monoclonal antibody with a high binding affinity for the extracellular domain of VEGFR-2.[64] Preclinical studies showed that targeting this VEGF family receptor was associated with inhibition of VEGF-mediated signaling, proliferation and migration of human endothelial cells and anti-tumor activity in animal models.[65,66,67,68] The results of two phase III clinical trials have recently confirmed that VEGFR-2 is a valuable therapeutic target in gastric cancer [Table 2].
Table 2
Phase II/III clinical trials of ramucirumab in gastric cancer
|
REGARD was a global, double-blind, placebo-controlled, phase III trial which randomized in a 2:1 ratio 355 chemorefractory metastatic gastric cancer patients to single agent ramucirumab or placebo.[69] The primary endpoint was OS. Median OS (5.2 months vs. 3.8 months, HR = 0.776, P = 0.047), median PFS (2.1 months vs. 1.3 months, HR = 0.483, P < 0 xss=removed>P < 0>
More recently, the benefit of the ramucirumab in the refractory setting has been confirmed in the RAINBOW trial, an international, multicentre, randomised phase III trial of weekly paclitaxel plus or minus ramucirumab.[70] The primary endpoint was OS and a total of 665 patients were enrolled. The addition of the ramucirumab to standard chemotherapy was demonstrated to improve OS from 7.36 months to 9.63 months (HR = 0.807, P = 0.0169). The study also met its secondary endpoints of PFS (2.86 vs. 4.40 months, HR = 0.635, P = 0.0001) and response rate (16% vs. 28%, P = 0.0001). When the survival outcomes are analyzed by geographical region, it appears evident that ramucirumab has similar activity in both Asian (33.5% of the study population) and Western patients (66.5% of the study population), with the impact of ramucirumab on OS in the former group being markedly diluted by the more favorable tumors phenotype and the increased use of treatments after study cessation.[71]
Safety analyses of these trials showed that the ramucirumab had a manageable safety profile. In the REGARD trial, the incidence of grade ≥3 adverse events was similar between the two arms and the use of ramucirumab was not associated with a deterioration of quality of life. In the RAINBOW trial the addition of ramucirumab to paclitaxel appeared to increase the risk of grade ≥3 chemotherapy-related toxicities including neutropenia (40.7% vs. 18.8%), fatigue (11.9% vs. 5.5%) and neuropathy (8.3% vs. 4.6%). In terms of adverse events of special interest for ramucirumab, only grade ≥3 hypertension was reported to be significantly more frequent in the investigational arm of both trials (8% vs. 3% in REGARD and 14.7%. vs 2.7% in RAINBOW).[69,70]
In contrast to the refractory setting, the addition of the ramucirumab to chemotherapy failed to show superiority over chemotherapy alone in the first-line setting. In a recent multicenter, double-blind, phase II trial, 168 patients with previously untreated unresectable locally advanced or metastatic esophageal, gastric or OGJ adenocarcinoma were randomized to receive mFOLFOX6 plus ramucirumab or placebo.[72] Although patients in the investigational arm experienced a higher disease control rate (85% vs. 67%, P = 0.008), no difference was observed in PFS (primary endpoint) (6.4 vs. 6.7 months, HR = 0.98, P = 0.89) and OS (11.7 vs. 11.5 months, HR = 1.08) between the two arms. Subgroup analyses suggest that the inclusion of patients with esophageal cancers (>45%) and the higher rate of treatment discontinuation before tumors progression in the investigational arm (27% vs. 10%) may have negatively influenced the results of the study.
Based on the positive results of the REGARD trial, in April 2014 ramucirumab has been granted FDA approval as second line treatment in patients with advanced or metastatic gastric or esophago-gastric junction cancers who progressed on fluoropyrimidine-or platinum-containing first-line chemotherapy.[73]
BIOMARKERS FOR ANTI-ANGIOGENIC THERAPIES IN GASTRIC CANCER
One of the reasons of failure (or limited benefit) of antiangiogenic agents in clinical trials of solid tumors is the unavailability of predictive biomarkers that may identify tumors that are more addicted to activated pro-angiogenic signaling pathways and therefore theoretically more sensitive to inhibitors of angiogenesis. In gastric cancer, data on the role of potential biomarkers are available only for bevacizumab and largely derive from the preplanned correlative analyses of the AVAGAST trial which included the prospective collection of tumor tissue and blood samples to evaluate both tissue and circulating biomarkers (including VEGF-A, VEGFR-1, VEGFR-2, NRP-1 and plasma VEGF-A).[74]
In this study, high levels of circulating VEGF-A and increased tumor expression of NRP-1 were found to be unfavorable prognostic factors associated with shorter survival in the placebo arm. More interestingly, the same biomarkers appeared to predict bevacizumab benefit in the investigational arm. The OS benefit of bevacizumab was found to be higher in patients with high VEGF-A levels (HR = 0.72) compared with patients with low VEGF-A levels (HR = 1.01) (interaction test P = 0.07) and in patients with low NRP-1 [removed]HR = 0.75) compared with patients with high NRP-1 [removed]HR = 1.07) (interaction test P = 0.06). Of note, the effect associated with the circulating levels of VEGF-A was evident only in patients from nonAsian-Pacific regions. Although interesting, these results remain hypothesis-generating and potentially biased by geographic differences in the process of tissue acquisition and the absence of standardized techniques and established cut-off points for evaluation of biomarker expression.[75]
To the best of our knowledge, there are no other published studies which investigated the potential association between tumors biomarkers and response to antiangiogenic agents in gastric cancer with the exception of an exploratory analysis of the previous small phase II trial of bevacizumab in combination with capecitabine and oxaliplatin. In this study, tumors expression of NRP-1 and NRP-2 was assessed and correlated with outcome.[60] Although the small sample size and the absence of a control group do not allow to draw any conclusion on the predictive effect of these biomarkers, a statistically significant association between high mRNA levels of NRP-2 and poor survival outcomes was observed. A similar negative prognostic effect was found for high mRNA levels of NRP-1. However, this was not statistically significant.
ANTI-ANGIOGENIC AGENTS UNDER INVESTIGATION
A number of antiangiogenic agents, some of which have already been approved for use in other tumors types, are currently under investigation in gastric cancer.
The most promising data regarding novel antiangiogenic drugs are on Apatinib which is an oral, small molecule tyrosin-kinase inhibitor targeting VEGFR-2. A phase III randomized, double-blind, placebo-controlled trial in chemorefractory gastric cancer patients has been recently presented at the 2014 ASCO Annual Meeting.[76] In this study, 273 Chinese patients who had previously progressed on second line therapy were randomised in a 2:1 ratio to apatinib or placebo. Median OS, the primary endpoint of the study, was significantly prolonged from 140 days with placebo to 195 days with apatinib (HR = 0.71, P < 0 xss=removed xss=removed>P < 0>
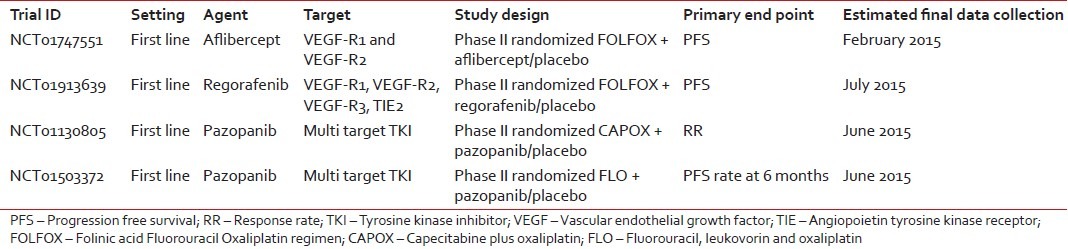
Less mature data are available for other antiangiogenic agents, including aflibercept and the multi-tyrosine kinase inhibitors regorafenib and pazopanib. These agents are currently being investigated in placebo-controlled phase II trials of first-line treatment [Table 3].
Table 3
Ongoing clinical trials of anti-angiogenetic agents in gastric cancer
|
DISCUSSION
Angiogenesis plays a major role in tumors development and progression, and preclinical data suggest that the inhibition of angiogenic signaling pathways may have an important therapeutic potential in gastric cancer. However, clinical trials have so far provided contradictory results, and the antitumour activity of antiangiogenic agents has not always translated into a significant survival benefit. Factors including heterogeneity of patient populations, ethnical differences in tumors biology and pharmacogenomics, drug mechanism of action, chemotherapy backbone and study design may explain the variable results observed with these agents in the clinical setting. Furthermore, in large trials, the absence of predictive biomarkers is likely to dilute any significant survival advantage, which may be associated with inhibition of angiogenesis in selected groups of patients.
In this review article, we have focused on the clinical efficacy of inhibitors of antiangiogenesis in gastric cancer. However, it is worth highlighting that a comprehensive appraisal of the role of these agents should also include the assessment of the key parameter such as cost-effectiveness. To our knowledge, there are no cost-effective analyses conducted in patients treated with bevacizumab or ramucirumab for advanced gastric cancer. We envisage that, based on the marginal survival improvement observed with inhibitors of angiogenesis in unselected populations, refinement of patient selection by virtue of molecular stratification will be crucial to meet the increasingly stringent criteria used by Healthcare Regulatory Agencies in the drug approval process.
Recently, investigators from the Cancer Genome Atlas Research Network have provided a comprehensive molecular characterization of gastric cancer.[77] Four molecular subtypes have been identified two of which, the chromosomically instable tumors and the genomically stable tumors, were associated with recurrent amplification of the VEGF-A gene and elevated expression of angiogenesis-related pathway, respectively. Although these data cannot yet influence the therapeutic strategies to use in selected individuals in routine practice, they can help to reveal which tumors are more addicted to activated angiogenic pathways and are hence more suitable for an investigational approach with antiangiogenic based therapies. Further studies, including prospective clinical trials with a treatment by biomarker interaction design, are certainly needed to identify and validate tumors tissue or circulating biomarkers that can be routinely used to predict treatment response.
ACKNOWLEDGMENTS
We acknowledge support from the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research and from the Peter Stebbings Memorial Charity.
Footnotes
Source of Support: We acknowledge support from the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research and from the Peter Stebbings Memorial Charity.
Conflict of Interest: None declared.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Available from: http://www.globocan.iarc.fr. [Last accessed on 2014 Aug 24].
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006;24:4991-7.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46.
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93.
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 2008;9:215-21.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97.
- Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44.
- Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: A meta-analysis. Ann Oncol 2013;24:2850-4.
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86.
- Burgos H. Angiogenic and growth factors in human amnio-chorion and placenta. Eur J Clin Invest 1983;13:289-96.
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 1977;14:53-65.
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014;17:471-94.
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604-13.
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989;161:851-8.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306-9.
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 2000;55:15-35.
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91.
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005;307:58-62.
- Smith NR, Baker D, Farren M, Pommier A, Swann R, Wang X, et al. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin Cancer Res 2013;19:6943-56.
- Ocaña A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: Similar results but different conclusions. J Clin Oncol 2011;29:254-6.
- Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993;53:4727-35.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 1996;56:2671-6.
- Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858-63.
- Maeda K, Kang SM, Ogawa M, Onoda N, Sawada T, Nakata B, et al. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial cell growth factor expression in gastric carcinoma. Int J Cancer 1997;74:545-50.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol 1997;15:826-32.
- Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, et al. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer 1999;86:566-71.
- Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res 1996;2:1679-84.
- Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, et al. Circulating VEGF levels in the serum of gastric cancer patients: Correlation with pathological variables, patient survival, and tumor surgery. Ann Surg 2002;236:37-42.
- Vidal O, Metges JP, Elizalde I, Valentíni M, Volant A, Molina R, et al. High preoperative serum vascular endothelial growth factor levels predict poor clinical outcome after curative resection of gastric cancer. Br J Surg 2009;96:1443-51.
- Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, et al. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol 2010;40:1147-53.
- Kim JG, Sohn SK, Chae YS, Cho YY, Bae HI, Yan G, et al. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with gastric cancer. Ann Oncol 2007;18:1030-6.
- Guan X, Zhao H, Niu J, Tan D, Ajani JA, Wei Q. Polymorphisms of TGFB1 and VEGF genes and survival of patients with gastric cancer. J Exp Clin Cancer Res 2009;28:94.
- Scartozzi M, Loretelli C, Galizia E, Mandolesi A, Pistelli M, Bittoni A, et al. Role of vascular endothelial growth factor (VEGF) and VEGF-R genotyping in guiding the metastatic process in pT4a resected gastric cancer patients. PLoS One 2012;7:e38192.
- Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Hwang JA, et al. The relationship of vascular endothelial growth factor gene polymorphisms and clinical outcome in advanced gastric cancer patients treated with FOLFOX: VEGF polymorphism in gastric cancer. BMC Cancer 2013;13:43.
- Semenza GL. Surviving ischemia: Adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 2000;106:809-12.
- Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res 2006;12:5112-7.
- Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, et al. Clinicopathologic significance of hypoxia-inducible factor 1alpha overexpression in gastric carcinomas. J Surg Oncol 2006;94:149-54.
- Ma J, Zhang L, Ru GQ, Zhao ZS, Xu WJ. Upregulation of hypoxia inducible factor 1alpha mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma. World J Gastroenterol 2007;13:1680-6.
- Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, et al. Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer 2008;8:123.
- Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, et al. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS One 2014;9:e90678.
- Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, et al. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst 2004;96:946-56.
- Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999;5:1823-9.
- Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, et al. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer 2001;37:918-23.
- Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer 2002;38:1413-9.
- Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol 2003;33:21-7.
- Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, et al. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 2005;115:388-92.
- Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: Two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006;24:228-40.
- Presta LG, Chen H, O′Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997;57:4593-9.
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671-80.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50.
- Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J Clin Oncol 2010;28:2144-50.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40.
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039-45.
- Shah MA, Ramanathan RK, Ilson DH, Levnor A, D′Adamo D, O′Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201-6.
- Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011;29:868-74.
- El-Rayes BF, Zalupski M, Bekai-Saab T, Heilbrun LK, Hammad N, Patel B, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol 2010;21:1999-2004.
- Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist 2013;18:271-2.
- Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76.
- Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2014.
- Okines AF, Langley RE, Thompson LC, Stenning SP, Stevenson L, Falk S, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: A safety report. Ann Oncol 2013;24:702-9.
- Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7.
- Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem 2003;278:43496-507.
- Miao HQ, Hu K, Jimenez X, Navarro E, Zhang H, Lu D, et al. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun 2006;345:438-45.
- Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer 2002;38:1133-40.
- Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia 2003;17:604-11.
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9.
- Wilke H, Van Cutsem E, Cheul Oh S, Bodoky G, Shimada Y, Hironaka S, et al., RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum-and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). ASCO Meeting Abstracts. J Clin Oncol 2014;32 3 Suppl:LBA7.
- Hironaka S, Shimada Y, Sugimoto N, Komatsu Y, Nishina T, Yamaguchi K et al., RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab (RAM) plus paclitaxel (PTX) versus placebo (PL) plus PTX in the treatment of metastatic gastroesophageal junction and gastric adenocarcinoma (mGC) following disease progression on first-line platinum-and fluoropyrimidine-containing combination therapy - Efficacy analysis in Japanese and Western patients. 2014 ASCO Annual Meeting. J Clin Oncol 2014;32:5S. [Suppl; abstr 4005].
- Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip AP, Cohnet LA, et al., Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): Randomized, double-blind, multicenter phase 2 trial. ASCO Meeting Abstracts. J Clin Oncol 2014;32 15 Suppl:4004.
- Poole RM, Vaidya A. Ramucirumab: First global approval. Drugs 2014;74:1047-58.
- Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27.
- Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: Are we there yet? Clin Cancer Res 2013;19:2824-7.
- Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. 2014 ASCO Annual Meeting. J Clin Oncol 2014;32 15 Suppl:5S. [Suppl; abstr 4003].
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Available from: http://www.globocan.iarc.fr. [Last accessed on 2014 Aug 24].
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006;24:4991-7.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46.
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93.
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 2008;9:215-21.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97.
- Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44.
- Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: A meta-analysis. Ann Oncol 2013;24:2850-4.
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86.
- Burgos H. Angiogenic and growth factors in human amnio-chorion and placenta. Eur J Clin Invest 1983;13:289-96.
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 1977;14:53-65.
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014;17:471-94.
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604-13.
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989;161:851-8.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306-9.
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 2000;55:15-35.
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91.
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005;307:58-62.
- Smith NR, Baker D, Farren M, Pommier A, Swann R, Wang X, et al. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin Cancer Res 2013;19:6943-56.
- Ocaña A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: Similar results but different conclusions. J Clin Oncol 2011;29:254-6.
- Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993;53:4727-35.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 1996;56:2671-6.
- Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858-63.
- Maeda K, Kang SM, Ogawa M, Onoda N, Sawada T, Nakata B, et al. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial cell growth factor expression in gastric carcinoma. Int J Cancer 1997;74:545-50.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol 1997;15:826-32.
- Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, et al. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer 1999;86:566-71.
- Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res 1996;2:1679-84.
- Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, et al. Circulating VEGF levels in the serum of gastric cancer patients: Correlation with pathological variables, patient survival, and tumor surgery. Ann Surg 2002;236:37-42.
- Vidal O, Metges JP, Elizalde I, Valentíni M, Volant A, Molina R, et al. High preoperative serum vascular endothelial growth factor levels predict poor clinical outcome after curative resection of gastric cancer. Br J Surg 2009;96:1443-51.
- Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, et al. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol 2010;40:1147-53.
- Kim JG, Sohn SK, Chae YS, Cho YY, Bae HI, Yan G, et al. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with gastric cancer. Ann Oncol 2007;18:1030-6.
- Guan X, Zhao H, Niu J, Tan D, Ajani JA, Wei Q. Polymorphisms of TGFB1 and VEGF genes and survival of patients with gastric cancer. J Exp Clin Cancer Res 2009;28:94.
- Scartozzi M, Loretelli C, Galizia E, Mandolesi A, Pistelli M, Bittoni A, et al. Role of vascular endothelial growth factor (VEGF) and VEGF-R genotyping in guiding the metastatic process in pT4a resected gastric cancer patients. PLoS One 2012;7:e38192.
- Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Hwang JA, et al. The relationship of vascular endothelial growth factor gene polymorphisms and clinical outcome in advanced gastric cancer patients treated with FOLFOX: VEGF polymorphism in gastric cancer. BMC Cancer 2013;13:43.
- Semenza GL. Surviving ischemia: Adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 2000;106:809-12.
- Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res 2006;12:5112-7.
- Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, et al. Clinicopathologic significance of hypoxia-inducible factor 1alpha overexpression in gastric carcinomas. J Surg Oncol 2006;94:149-54.
- Ma J, Zhang L, Ru GQ, Zhao ZS, Xu WJ. Upregulation of hypoxia inducible factor 1alpha mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma. World J Gastroenterol 2007;13:1680-6.
- Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, et al. Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer 2008;8:123.
- Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, et al. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS One 2014;9:e90678.
- Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, et al. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst 2004;96:946-56.
- Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999;5:1823-9.
- Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, et al. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer 2001;37:918-23.
- Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer 2002;38:1413-9.
- Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol 2003;33:21-7.
- Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, et al. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 2005;115:388-92.
- Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: Two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006;24:228-40.
- Presta LG, Chen H, O′Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997;57:4593-9.
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671-80.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50.
- Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J Clin Oncol 2010;28:2144-50.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40.
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039-45.
- Shah MA, Ramanathan RK, Ilson DH, Levnor A, D′Adamo D, O′Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201-6.
- Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011;29:868-74.
- El-Rayes BF, Zalupski M, Bekai-Saab T, Heilbrun LK, Hammad N, Patel B, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol 2010;21:1999-2004.
- Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist 2013;18:271-2.
- Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76.
- Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2014.
- Okines AF, Langley RE, Thompson LC, Stenning SP, Stevenson L, Falk S, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: A safety report. Ann Oncol 2013;24:702-9.
- Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7.
- Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem 2003;278:43496-507.
- Miao HQ, Hu K, Jimenez X, Navarro E, Zhang H, Lu D, et al. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun 2006;345:438-45.
- Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer 2002;38:1133-40.
- Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia 2003;17:604-11.
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9.
- Wilke H, Van Cutsem E, Cheul Oh S, Bodoky G, Shimada Y, Hironaka S, et al., RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum-and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). ASCO Meeting Abstracts. J Clin Oncol 2014;32 3 Suppl:LBA7.
- Hironaka S, Shimada Y, Sugimoto N, Komatsu Y, Nishina T, Yamaguchi K et al., RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab (RAM) plus paclitaxel (PTX) versus placebo (PL) plus PTX in the treatment of metastatic gastroesophageal junction and gastric adenocarcinoma (mGC) following disease progression on first-line platinum-and fluoropyrimidine-containing combination therapy - Efficacy analysis in Japanese and Western patients. 2014 ASCO Annual Meeting. J Clin Oncol 2014;32:5S. [Suppl; abstr 4005].
- Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip AP, Cohnet LA, et al., Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): Randomized, double-blind, multicenter phase 2 trial. ASCO Meeting Abstracts. J Clin Oncol 2014;32 15 Suppl:4004.
- Poole RM, Vaidya A. Ramucirumab: First global approval. Drugs 2014;74:1047-58.
- Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27.
- Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: Are we there yet? Clin Cancer Res 2013;19:2824-7.
- Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. 2014 ASCO Annual Meeting. J Clin Oncol 2014;32 15 Suppl:5S. [Suppl; abstr 4003].
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9.


 PDF
PDF  Views
Views  Share
Share

