Antibody Drug Conjugates
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(06): 889-892
DOI: DOI: 10.4103/ijmpo.ijmpo_313_20
Abstract
Antibody drug conjugates (ADCs) are chemically engineered drugs consisting of monoclonal antibody (mAb) and cytotoxic compound attached chemically by a linker. Upon attachment to a specific target antigen, ADC enters into the cell and payload is released, which finally leads to cell killing. Payloads are broadly divided into tubulin-disrupting agents and DNA-damaging agents. Most of the current ADCs utilize humanized mAbs, and fully human mAbs are under investigation. ADC development process is accelerated by better designing and bio-engineering methods.
Publication History
Received: 29 June 2020
Accepted: 19 August 2020
Article published online:
14 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Antibody drug conjugates (ADCs) are chemically engineered drugs consisting of monoclonal antibody (mAb) and cytotoxic compound attached chemically by a linker. Upon attachment to a specific target antigen, ADC enters into the cell and payload is released, which finally leads to cell killing. Payloads are broadly divided into tubulin-disrupting agents and DNA-damaging agents. Most of the current ADCs utilize humanized mAbs, and fully human mAbs are under investigation. ADC development process is accelerated by better designing and bio-engineering methods.
Introduction
As per the Globocan 2018 data, approximately 1.1 million new cancer cases were diagnosed in India.[1] Cancer figures in the top five causes of mortality in India and is the second most common cause of death in the West.[2] For several decades, cancer therapy primarily consisted of surgery, radiation, and chemotherapy. With the availability of technological advancements in science, new classes of drugs such as antibody drug conjugates (ADCs), monoclonal antibodies, small-molecule kinase inhibitors, and immune checkpoint inhibitors have emerged. Conventional cytotoxic therapy carries certain unwanted toxicities by acting on normally proliferating cells such as bone marrow, mucosal lining, and hair follicle. To circumvent these off-target effects, a new class of drugs called ADCs were developed. ADCs ensure targeted drug delivery by a combination of monoclonal antibody (mAb) and cytotoxic chemotherapy moiety (payload), which is joined by a chemical linker.
The German scientist Paul Ehrlich proposed the concept of “magic bullets” a century ago, describing them as those that identify the target without harming the organism.[3] The legacy of discovery continued to evolve into various clinical applications including the ADC discovery. Though the ADC experiments date back to the 1980s, they have become more popular in recent times.[4] Initial studies of ADC are related to methotrexate attached to an antibody by an ester conjugate to target specific cell lineage; it demonstrated good efficacy in vitro and in vivo. In the 1990s, the first ADC using chimeric and humanized monoclonal antibodies were tested.[5]
Structural Components of Antibody Drug Conjugate
The main components of ADC are antibody moiety, linker, and payload (chemotherapeutic agent). These three components have been discussed below.
Antibody
The antibody moiety is designed to target the specific tumor-associated antigen. Its primary function is to attach to a specific target and ultimately lead to internalization of the antigen–antibody complex, further leading to delivery of the payload intracellularly. There are more than 300 unique antigens described for therapeutic target.[6] Some of the tumor-associated target antigens expressed in various cancers are listed in [Table 1].
|
List of various target antigens and cancers |
Cancer |
|---|---|
|
CD33 |
Acute myeloid leukemia |
|
CD30 |
Classical Hodgkin lymphoma, anaplastic large cell lymphoma |
|
CD22 |
Acute lymphoblastic leukemia, non-Hodgkin lymphoma, chronic lymphocytic leukemia |
|
Her-2 |
Breast cancer |
|
EGFR |
Non-small cell lung cancer, glioblastoma multiforme |
|
CD70 |
Non-Hodgkin lymphoma, renal cell carcinoma |
|
CD19 |
B-cell leukemias |
|
Mesothelin |
Malignant pleural mesothelioma |
|
PSMA |
Prostate cancer |
|
CD37 |
B-cell leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma |
|
DLL-3 |
Small cell lung cancer |
|
CD138 |
Multiple myeloma |
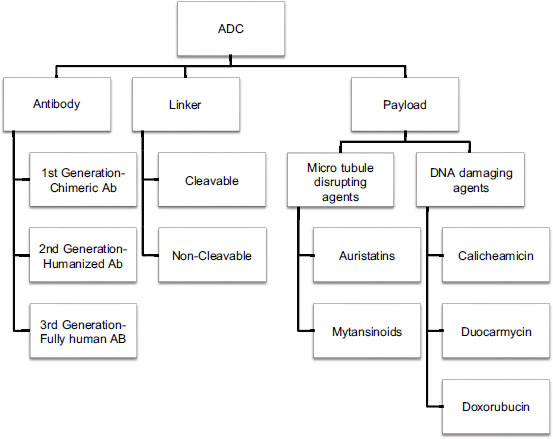
| Figure.1:Comprehensive diagram of antibody drug conjugates
Mechanism of Action of Antibody Drug Conjugate
ADCs are given intravenously to prevent degradation of the mAb by gastric enzymes. The prerequisite for action of ADC is preferential expression of specific target on tumor tissue for selective entry into the cell. The mechanism is illustrated in [Figure 2]. Antibody-dependent cytotoxicity is a major mechanism of cell death in mAb-based therapy which is typically not observed in ADC action.{Figure 2}
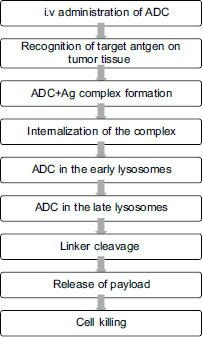
| Figure.2:Mechanism of action of antibody drug conjugate
Bystander Killing
When the drug from ADC is released within the extracellular space or from target cell (following internalization and degradation of ADC), surrounding or bystander cells take up the drug and could be killed. This is known as bystander killing. The surrounding cells may or may not express the ADC target antigen. Bystander killing mediated by ADC depends on factors such as the extent of ADC internalization after binding to the target antigen, the type of linker (cleavable or noncleavable), and hydrophobicity of cytotoxic agent.[13] ADCs containing cleavable linker may have more bystander killing compared to ADCs with noncleavable linker.
Resistance Mechanisms against Antibody Drug Conjugate
There are several proposed mechanisms by which resistance to ADC occurs.[14] Although there is no single postulated way, most often, there will be multiple pathways. These mechanisms include:
-
Alterations of target antigen
-
Interference with ADC internalization
-
Changes in trafficking pathway(s)
-
Modification of cell cycle and its regulators
-
Activation of drug efflux pump
-
Apoptotic dysregulation.
The list of approved ADCs and their indications is given in [Table 2].
|
Drug |
Target Ag |
Linker |
Payload |
Indication |
Year of approval |
Dose |
Side effects |
|---|---|---|---|---|---|---|---|
|
MMAE: Monomethyl auristatin E, ALCL: Anaplastic large cell lymphoma, AML: Acute myeloid leukemia, ALL: Acute lymphoblastic leukemia, DLBCL: Diffuse large B-cell lymphoma, TNBC: Triple-negative breast cancer, LFT: Liver function test, LV: Left ventricular |
|||||||
|
Gemtuzumab ozogamicin |
CD33 |
Hydrazone cleavable |
Calicheamicin |
CD33+AML |
2017 |
3 mg/m2 D1, D4, D7 |
Neutropenia, thrombocytopenia, infusion reactions, venoocclusive disease |
|
Iotuzumab ozogamicin |
CD22 |
Acid-labile cleavable |
Calichemicin |
R/R ALL |
2017 |
0.8 mg/m2 D1 then 0.5 mg/m2 D8, D15 |
Fever, thrombocytopenia, neutropenia, LFT derangements |
|
Brentuximab vedotin |
CD30 |
Protease cleavable |
MMAE |
cHL, ALCL |
2018 |
1.2 mg/kg every 2 as 1st-line therapy, 1.8 mg/ kg every 3 weekly for relapsed cHL and ALCL |
Nausea, neuropathy, neutropenia, infection |
|
T-DM1 |
Her2neu |
Thioether noncleavable linker |
Mertansine |
Her2+breast cancer |
2019 |
3.6 mg/kg every 3 weekly |
Thrombocytopenia, fatigue, LFT derangements, anemia |
|
Polatuzumab vedotin |
CD79b |
Protease cleavable |
MMAE |
R/R DLBCL |
2019 |
1.8 mg/kg every 3 weekly |
Neuropathy, neutropenia, thrombocytopenia, progressive multifocal leukoencephalopathy, tumor lysis syndrome |
|
Trastuzumab deruxtecan |
Her2neu |
Peptide cleavable |
Dxd (topoisomerase 1 inhibitor) |
Her2+breast cancer |
2019 |
5.4 mg/kg every 3 weekly |
Interstitial lung disease, LV dysfunction |
|
Enfortumab vedotin |
Nectin-4 |
Protease cleavable |
MMAE |
Urothelial carcinoma |
2019 |
1.25 mg/kg D1, D8, D15 every 4 weekly |
Hyperglycemia, neuropathy, skin reaction, extravasation reaction |
|
Sacituzumab govitecan |
Trop-2 |
Hydrolysable cleavable |
SN-38 Metastatic (topoisomerase TNBC 1 inhibitor) |
2020 |
10 mg/kg D1, D8 every 3 weekly |
Nausea, neutropenia, anemia, vomiting, diarrhea, fatigue |
|

| Figure.1:Comprehensive diagram of antibody drug conjugates

| Figure.2:Mechanism of action of antibody drug conjugate
References
- 356-india-fact-sheets.pdf. Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. [Last accessed on 2020 Jun 03].
- Census of India Website: Causes of Death Statistics. Available from: https://censusindia.gov.in/vital_statistics/causesofdeath.html. [Last accessed on 2020 May 23].
- Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer 2008; 8: 473-80
- Kanellos J, Pietersz GA, McKenzie IF. Studies of methotrexate-monoclonal antibody conjugates for immunotherapy. JNCI J Natl Cancer Inst 1985; 75: 319-32
- Trail PA, Willner D, Lasch SJ, Henderson AJ, Hofstead S, Casazza AM. et al. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science 1993; 261: 212-5
- Strohl WR. Current progress in innovative engineered antibodies. Protein Cell 2018; 9: 86-120
- Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody–drug conjugates: A comprehensive review. Mol Cancer Res 2020; 18: 3-19
- Hughes B. Antibody-drug conjugates for cancer: Poised to deliver?. Nat Rev Drug Discov 2010; 9: 665-7
- Casi G, Neri D. Noninternalizing targeted cytotoxics for cancer therapy. Mol Pharm 2015; 12: 1880-4
- Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ. et al. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem 2008; 19: 759-65
- Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell 2018; 9: 33-46
- Nasiri H, Valedkarimi Z, Aghebati-Maleki L, Majidi J. Antibody-drug conjugates: Promising and efficient tools for targeted cancer therapy. J Cell Physiol 2018; 233: 6441-57
- Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br J Cancer 2017; 117: 1736-42
- García-Alonso S, Ocaña A, Pandiella A. Resistance to antibody-drug conjugates. Cancer Res 2018; 78: 2159-65


 PDF
PDF  Views
Views  Share
Share

