Assessment of Angiogenesis in Children with Acute Lymphoblastic Leukemia Based on Serum Vascular Endothelial Growth Factor Assay
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 321-325
DOI: DOI: 10.4103/ijmpo.ijmpo_109_17
Abstract
Introduction: Vascular endothelial growth factor A (VEGFA) is a key proangiogenic cytokine. The role of angiogenesis in acute lymphoblastic leukemia (ALL) is still unclear. The purpose of the study was to assess angiogenesis in children with ALL based on serum VEGFA level determined at diagnosis and at remission with further participant subdivision into different risk groups. Materials and Methods: Forty children, aged 3–12 years (mean age: 8 years) with newly diagnosed ALL, were enrolled in the study. The control group (Group C) was twenty healthy children. According to the risk assessment, they were classified into a standard-risk group, an intermediate-risk group (IRG), or a high-risk group (HRG). Results: The median serum VEGFA levels at diagnosis were significantly higher in IRG and HRG as compared to Group C. The VEGFA levels at remission were significantly higher in all study groups, as compared to Group C. The differences in median values of serum VEGFA levels between the study groups both at diagnosis and at remission were not statistically significant. Conclusions: The angiogenesis in ALL seems to be intensified at diagnosis as a result of neoplasmatic bone marrow rebuilding and at remission as its intensive recovering.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Vascular endothelial growth factor A (VEGFA) is a key proangiogenic cytokine. The role of angiogenesis in acute lymphoblastic leukemia (ALL) is still unclear. The purpose of the study was to assess angiogenesis in children with ALL based on serum VEGFA level determined at diagnosis and at remission with further participant subdivision into different risk groups.
Materials and Methods:
Forty children, aged 3–12 years (mean age: 8 years) with newly diagnosed ALL, were enrolled in the study. The control group (Group C) was twenty healthy children. According to the risk assessment, they were classified into a standard-risk group, an intermediate-risk group (IRG), or a high-risk group (HRG).
Results:
The median serum VEGFA levels at diagnosis were significantly higher in IRG and HRG as compared to Group C. The VEGFA levels at remission were significantly higher in all study groups, as compared to Group C. The differences in median values of serum VEGFA levels between the study groups both at diagnosis and at remission were not statistically significant.
Conclusions:
The angiogenesis in ALL seems to be intensified at diagnosis as a result of neoplasmatic bone marrow rebuilding and at remission as its intensive recovering.
Introduction
Angiogenesis is a process essential for growth and development of all human tissues – both healthy and changed morbidly. There are a number of factors which stimulate angiogenesis, and the vascular endothelial growth factor A (VEGFA) has a key role among them. It was proved to be released by endothelial cells, lymphocytes, fibroblasts, macrophages, megakaryocytes, and tumor cells.[1,2,3] Whereas the importance of neovascularization and angiogenesis for solid tumors and their growth is understandable, the role of these processes in hematopoietic malignancies, especially acute lymphoblastic leukemia (ALL), has still been debated. Some researchers state that proangiogenic cytokines of leukemic origin stimulate angiogenesis in patients with ALL.[4,5] The leukemic cells which have VEGFA surface receptors may be susceptible to both autocrine and paracrine stimulation.[5] This promotes both the blood vessel formation within the bone marrow and the proliferation of malignant cells. On the other hand, there are studies showing that leukemic cell clones inhibit angiogenesis and destroy normal progenitor bone marrow cells responsible mainly for VEGFA production.[6,7] The described discrepant opinions justify the need of further research on angiogenesis in ALL.
The purpose of the study was to assess angiogenesis in children with ALL based on serum VEGFA level determined at diagnosis and at remission (day 33 of treatment), with further participant subdivision into different risk groups.
Materials and Methods
Procedure
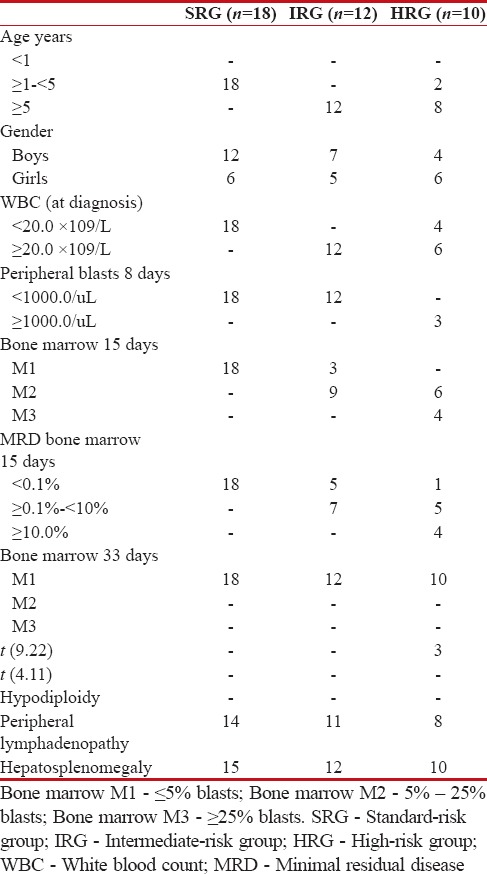
Forty children (23 boys – 57.5%; 17 girls – 42.5%) aged 3–12 years (mean age: 8 years), with newly diagnosed ALL, were enrolled in the study. All participants were treated according to the ALL Intercontinental 2002 (ALL IC 2002) protocol. They were classified according to the risk into one of the three groups: a standard-risk group (SRG; n = 18), an intermediate-risk group (IRG; n = 12), and a high-risk group (HRG; n = 10). The risk assessment included patient age at diagnosis, white blood count at diagnosis, blast cell count per 1 μL of peripheral blood on day 8 of treatment, blast cell count (%) in bone marrow on day 15 and 33 of treatment, minimal residual disease on day 15 as well as cytogenetic and biomolecular investigation findings [Table 1].
Table 1
The clinical characteristic examinated groups
|
The exclusion criteria were children with known additional physiological and pathological processes such as menstruation, previous injuries, or surgery, which could further enhance angiogenesis.
The control group (Group C) consisted of 20 healthy children and patients from pediatric clinic with functional gastrointestinal tract disturbances (13 boys – 65%, 7 girls – 35%) aged 2–15 years (mean age: 8 years).
The serum level of VEGFA was determined at diagnosis and at remission.
Fasting blood samples for laboratory analyses were collected from the ulnar vein using a G0.8 needle, after prior skin disinfection with 70% alcohol. To assess the serum VEGF concentration, additional 2 cm3 blood samples were collected from each participant/control during a routine phlebotomy. Clotted samples were centrifuged for 20 min at 2000 rpm. The obtained serum samples were frozen at −80°C. The analyses were performed using the Human VEGFA Quantikine Colorimetric Sandwich ELISA kit (R&D Systems).
The serum VEGFA levels in study participants were compared with those of the controls.
All participants and controls as well as their parents/carers were provided detailed and sufficient information on the method and purpose of the study. A written informed consent was obtained from parents for collecting an additional 2 cm3 blood sample for observational research purposes.
Statistical analysis
Since the distribution of variables was considerably different from the normal distribution (Kolmogorov–Smirnov test), they were described using the median (range); the hypotheses were verified using the nonparametric tests. The differences between the two groups in relation to individual numerical variables were verified using the Mann–Whitney U-test. P < 0>
Results
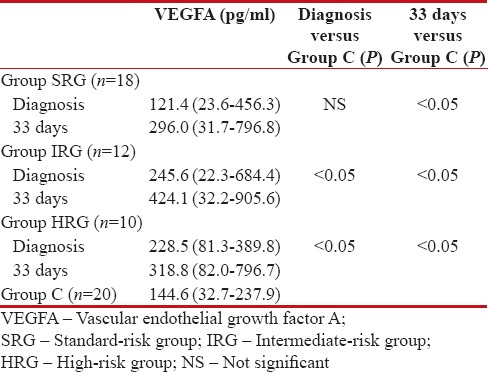
The median of serum VEGFA at diagnosis was comparable between the SRG and the Group C and was significantly higher in IRG and HRG as compared to the Group C. At remission, the median of serum VEGFA level was significantly higher in all study groups, as compared to the Group C [Table 2].
Table 2
The median values of serum vascular endothelial growth factor levels in the examinated groups and control group
|
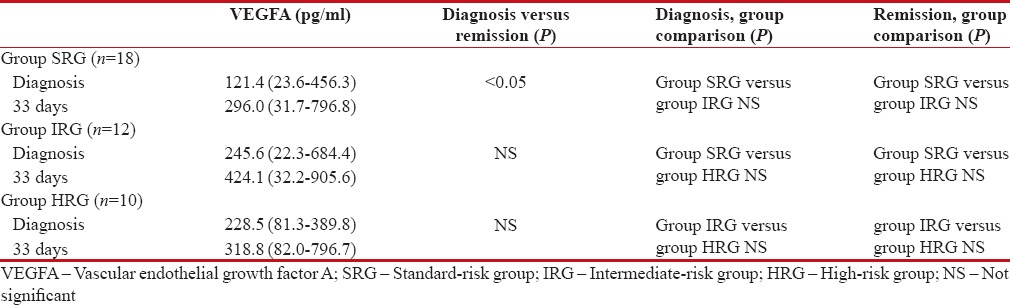
The median of serum VEGFA levels in SRG was significantly higher at remission, as compared to diagnosis. The median values of serum VEGFA at remission were also higher compared to diagnosis in IRG and HRG, but the differences were not statistically significant [Table 3].
Table 3
The median values of serum vascular endothelial growth factor levels in the groups both at diagnosis and at remission
|
The differences in median values of serum VEGFA levels between the groups both at diagnosis and at remission were not statistically significant [Table 3].
Discussion
The role of angiogenesis in cancer has been studied for many years. It can be assessed by means of different markers, for example, serum, plasma, or urine concentration of proangiogenic cytokines, the expression of proangiogenic cytokines and their receptors (both surface and soluble ones) determined using the polymerase chain reaction (PCR), as well as the microvascular density (MVD) count which reflects changes in blood vessel formation.[8,9,10,11]
Progenitor cells, endothelium, and stroma of normal bone marrow were shown to express proangiogenic cytokines.[7,10,12] The said cytokines affect stem cells and entire bone marrow microenvironment by both autocrine and paracrine stimulation.[13,14] The megakaryocyte-rich areas of human bone marrow present with particularly high MVD and VEGF mRNA level.[10]
Based on the above data, a hypothesis was postulated that the level of the key angiogenesis triggering cytokine, i.e., VEGFA, was low in patients with ALL due to the destruction of both bone marrow stem cells and bone marrow microenvironment by the clones of leukemic cells.[6] Others, however, hypothesized that the existence of soluble VEGF receptor in human serum and the presence of VEGF receptor on leukemic cells may induce their interaction, thus decreasing serum VEGF level.[7,15]
This hypothesis was confirmed by Kalra et al.,[7] who showed significantly lower VEGF levels at diagnosis as compared to remission in thirty children with ALL. In addition, according to them, low VEGF levels in pediatric nonresponders to treatment may, for instance, reflect the high expression of VEGF receptor on leukemic cells. As a result, more VEGF is bound to the receptors and its serum level decreases.[7] Similarly, Yetgin et al.[16] and Aref et al.[17] confirmed significantly lower VEGF levels at diagnosis as compared to remission in their studies. Our research appears to reproduce their findings. We showed lower serum VEGFA median at diagnosis as compared to remission. The observed difference was statistically significant only in the SRG. In two other groups – IRG and HRG, serum VEGFA levels were also lower at diagnosis as compared to remission, but the differences were not statistically significant.
According to the research carried out by the Children's Oncology Group,[18] the longest event-free survival was observed in those children with ALL, who presented with lower VEGFA levels both at diagnosis and at the remission. In our research, the SRG participants with most favorable prognosis actually presented with the lowest VEGFA levels at diagnosis and at remission, as compared to other groups with poorer prognosis (IRG, HRG). At remission, median VEGFA in SRG was significantly higher as compared to diagnosis, which may be consistent with intensive bone marrow rebuilding and VEGFA production by the progenitor cells without its simultaneous uptake by malignant cell receptors.
However, there are studies reporting contradictory findings. Chand et al.[19] who studied angiogenesis in a group of 15 children with ALL showed significantly higher serum VEGF level and MVD determined at diagnosis, as compared to the same values at remission. According to the report by Perez-Atayde et al.[20] published in 1997, the bone marrow of children with ALL presented with increased microvascular density (MVD). Hussong et al.[21] reported similar findings evaluating that bone and marrow specimens obtained from children with leukemia. According to Chand et al.,[21] patients with different hematologic malignancies, including ALL, who present with elevated MVD and VEGF at baseline, may make good candidates for antiangiogenic therapy, especially if they are poor responders to the first-line treatment.
There are studies which showed a lack of differences between angiogenesis biomarker levels in patients with ALL at different stages of treatment. Leblebisatan et al.[8] used PCR to assess VEGF expression in leukemic cells of 28 children. They did not observe significant differences between the assays at diagnosis and recurrence at remission.
Our research points to the increased angiogenesis in ALL both at diagnosis and at remission, with its completely different triggers implicated at different time points. Significantly lower VEGFA values in healthy children as compared to the IRG and HRGs suggest the role of leukemic cells in VEGFA production. Higher VEGFA levels at remission in all children with ALL as compared to healthy controls may be indicative of intensive bone marrow regeneration and VEGFA production by normal bone marrow progenitor cells.
Our findings as well as other studies of this case sufficiently prove that angiogenesis in children with ALL has not been fully explained yet, so further research in large, uniform pediatric population is legitimate and essential. According to some researchers, serum levels of proangiogenic cytokines in patients newly diagnosed with ALL may be seen as a source of knowledge on its etiopathogenesis, which could later help monitor treatment response or affect treatment choices.[8,13,14]
Conclusions
The significantly higher level of serum VEGF in children with ALL in comparison with healthy control, both at the diagnosis and in the remission, could suggest the intensification of angiogenesis in the bone marrow, at diagnosis due to neoplasmatic proliferation, at remission due to physiological, intensive bone marrow rebuilding.
There is a tendency for higher serum VEGF level in the groups with less satisfying prognosis, which suggests further examinations on wider groups of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 1999;59:728-33.
- Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, et al. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study. Angiogenesis 2001;4:37-43.
- Fukushima N, Satoh T, Sano M, Tokunaga O. Angiogenesis and mast cells in non-Hodgkin's lymphoma: A strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma 2001;42:709-20.
- Medinger M, Passweg J. Role of tumour angiogenesis in haematological malignancies. Swiss Med Wkly 2014;144:w14050.
- Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 2001;276:3222-30.
- Mellgren K, Hedegaard CJ, Schmiegelow K, Müller K. Plasma cytokine profiles at diagnosis in pediatric patients with non-Hodgkin lymphoma. J Pediatr Hematol Oncol 2012;34:271-5.
- Kalra M, Dinand V, Choudhary S, Sachdeva A, Yadav SP. Serum vascular endothelial growth factor-a levels during induction therapy in children with acute lymphoblastic leukemia. Indian Pediatr 2013;50:659-62.
- Leblebisatan G, Antmen B, Sasmaz I, Kilinç Y. Vascular endothelial growth factor levels in childhood acute lymphoblastic and myeloblastic leukemia. Indian J Hematol Blood Transfus 2012;28:24-8.
- Koomagi R, Zintl F, Sauerbrey A, Volm M. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clin Cancer Res 2001;7:3381-4.
- Pruneri G, Bertolini F, Soligo D, Carboni N, Cortelezzi A, Ferrucci PF, et al. Angiogenesis in myelodysplastic syndromes. Br J Cancer 1999;81:1398-401.
- Aguayo A, Kantarjian HM, Estey EH, Giles FJ, Verstovsek S, Manshouri T, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer 2002;95:1923-30.
- Sensebe L, Deschaseaux M, Li J, Herve P, Charbord P. The broad spectrum of cytokine gene expression by myoid cells from the human marrow microenvironment. Stem Cells 1997;15:133-43.
- Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Serum cytokine and adhesion molecule profile differs in newly diagnosed acute myeloid and lymphoblastic leukemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:299-301.
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J Clin Oncol 2011;29:591-9.
- Hu Q, Dey AL, Yang Y, Shen Y, Jilani IB, Estey EH, et al. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer 2004;100:1884-91.
- Yetgin S, Yenicesu I, Cetin M, Tuncer M. Clinical importance of serum vascular endothelial and basic fibroblast growth factors in children with acute lymphoblastic leukemia. Leuk Lymphoma 2001;42:83-8.
- Aref S, Salama O, Shamaa S, El-Refaie M, Mourkos H. Angiogenesis factor pattern differs in acute lymphoblastic leukemia and chronic lymphocytic leukemia. Hematology 2007;12:319-24.
- Avramis IA, Panosyan EH, Dorey F, Holcenberg JS, Avramis VI; Children's Oncology Group. Correlation between high vascular endothelial growth factor-A serum levels and treatment outcome in patients with standard-risk acute lymphoblastic leukemia: A report from children's oncology group study CCG-1962. Clin Cancer Res 2006;12:6978-84.
- Chand R, Chandra H, Chandra S, Verma SK. Role of microvessel density and vascular endothelial growth factor in angiogenesis of hematological malignancies. Bone Marrow Res 2016;2016:5043483.
- Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997;150:815-21.
- Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 2000;95:309-13.
References
- Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 1999;59:728-33.
- Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, et al. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study. Angiogenesis 2001;4:37-43.
- Fukushima N, Satoh T, Sano M, Tokunaga O. Angiogenesis and mast cells in non-Hodgkin's lymphoma: A strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma 2001;42:709-20.
- Medinger M, Passweg J. Role of tumour angiogenesis in haematological malignancies. Swiss Med Wkly 2014;144:w14050.
- Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 2001;276:3222-30.
- Mellgren K, Hedegaard CJ, Schmiegelow K, Müller K. Plasma cytokine profiles at diagnosis in pediatric patients with non-Hodgkin lymphoma. J Pediatr Hematol Oncol 2012;34:271-5.
- Kalra M, Dinand V, Choudhary S, Sachdeva A, Yadav SP. Serum vascular endothelial growth factor-a levels during induction therapy in children with acute lymphoblastic leukemia. Indian Pediatr 2013;50:659-62.
- Leblebisatan G, Antmen B, Sasmaz I, Kilinç Y. Vascular endothelial growth factor levels in childhood acute lymphoblastic and myeloblastic leukemia. Indian J Hematol Blood Transfus 2012;28:24-8.
- Koomagi R, Zintl F, Sauerbrey A, Volm M. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clin Cancer Res 2001;7:3381-4.
- Pruneri G, Bertolini F, Soligo D, Carboni N, Cortelezzi A, Ferrucci PF, et al. Angiogenesis in myelodysplastic syndromes. Br J Cancer 1999;81:1398-401.
- Aguayo A, Kantarjian HM, Estey EH, Giles FJ, Verstovsek S, Manshouri T, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer 2002;95:1923-30.
- Sensebe L, Deschaseaux M, Li J, Herve P, Charbord P. The broad spectrum of cytokine gene expression by myoid cells from the human marrow microenvironment. Stem Cells 1997;15:133-43.
- Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Serum cytokine and adhesion molecule profile differs in newly diagnosed acute myeloid and lymphoblastic leukemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:299-301.
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J Clin Oncol 2011;29:591-9.
- Hu Q, Dey AL, Yang Y, Shen Y, Jilani IB, Estey EH, et al. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer 2004;100:1884-91.
- Yetgin S, Yenicesu I, Cetin M, Tuncer M. Clinical importance of serum vascular endothelial and basic fibroblast growth factors in children with acute lymphoblastic leukemia. Leuk Lymphoma 2001;42:83-8.
- Aref S, Salama O, Shamaa S, El-Refaie M, Mourkos H. Angiogenesis factor pattern differs in acute lymphoblastic leukemia and chronic lymphocytic leukemia. Hematology 2007;12:319-24.
- Avramis IA, Panosyan EH, Dorey F, Holcenberg JS, Avramis VI; Children's Oncology Group. Correlation between high vascular endothelial growth factor-A serum levels and treatment outcome in patients with standard-risk acute lymphoblastic leukemia: A report from children's oncology group study CCG-1962. Clin Cancer Res 2006;12:6978-84.
- Chand R, Chandra H, Chandra S, Verma SK. Role of microvessel density and vascular endothelial growth factor in angiogenesis of hematological malignancies. Bone Marrow Res 2016;2016:5043483.
- Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997;150:815-21.
- Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 2000;95:309-13.


 PDF
PDF  Views
Views  Share
Share

