Blastic Plasmacytoid Dendritic Cell Neoplasm: A Rare Case Report with Literature Review
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2021; 42(05): 496-500
DOI: DOI: 10.1055/s-0041-1736432
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematopoietic neoplasm for which there are no effective therapies. We present a 70-year-old male patient with multiple reddish painless, nonpruritic, and nonpedunculated nodules over the trunk, forearm, and thighs for a duration of 3 months. The nodules measured 0.5 to 2?cm in diameter. The peripheral smear findings were within normal limits. Excision biopsy was performed. Histomorphology and immunohistochemistry (CD123, CD 56, CD4, HLA-DR, CD43, and CD68) confirmed the diagnosis of BPDCN. Findings of marrow aspiration, biopsy and imaging studies were within normal limits. Patient demonstrated a good response with complete disappearance of all nodules by initial 2 weeks of therapy with a modified Berlin?Frankfurt?Munster (BFM) acute lymphoblastic leukemia (ALL) protocol and has completed 8 doses (LSAP [lincosamides, streptogramins A and pleuromutilins chemotherapy], 5,000 units/m2). The patient tolerated protocol extremely well.
Keywords
blastic plasmacytoid dendritic cell neoplasm - chemotherapy - stem cell therapy - targeted therapyEthics Statement
The authors hereby declare that Institutional Ethics Committee did not mandate IEC review for this study. The collection of data from electronic medical record (EMR) and laboratory reports do not require an informed consent form as per the hospital's standard operating procedures.
*?Authors have equally contributed for writing, review and editing the case report.
Publication History
Publication Date:
05 December 2021 (online)
? 2021. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematopoietic neoplasm for which there are no effective therapies. We present a 70-year-old male patient with multiple reddish painless, nonpruritic, and nonpedunculated nodules over the trunk, forearm, and thighs for a duration of 3 months. The nodules measured 0.5 to 2?cm in diameter. The peripheral smear findings were within normal limits. Excision biopsy was performed. Histomorphology and immunohistochemistry (CD123, CD 56, CD4, HLA-DR, CD43, and CD68) confirmed the diagnosis of BPDCN. Findings of marrow aspiration, biopsy and imaging studies were within normal limits. Patient demonstrated a good response with complete disappearance of all nodules by initial 2 weeks of therapy with a modified Berlin?Frankfurt?Munster (BFM) acute lymphoblastic leukemia (ALL) protocol and has completed 8 doses (LSAP [lincosamides, streptogramins A and pleuromutilins chemotherapy], 5,000 units/m2). The patient tolerated protocol extremely well.
Keywords
blastic plasmacytoid dendritic cell neoplasm - chemotherapy - stem cell therapy - targeted therapyIntroduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic malignancy with an incidence of 0.000045% and constitutes less than 1% of all acute leukemia.[1] This neoplasm predominantly affects elderly males with the median age being in the sixth decade of life and sex ratio of 3:1.[2] [3] It remained an orphan disease for long until recently when few series have been published with predominant cutaneous involvement making it difficult to establish clinical, biological, and prognostic features.[4] We present this rare case and discuss the diagnostic approach with various treatment options available for this entity.
Case Report
A 70-year-old male patient presented with insidious onset of multiple reddish painless nodules all over the body for the last 3 months. The nodules were of varying sizes ranging from 0.5 to 2?cm in diameter. The lesions started in the chest region, later spread over forearm, arm, thighs and then all over the body. Nodules were non-pruritic, nontender, and nonpedunculated. Clinically, no palpable lymphadenopathy or organomegaly was noted. The Eastern Cooperative Oncology Group (ECOG)/Karnofsky's index of performance status (KPS) performance score was 1. Hemogram was within normal limits with hemoglobin of 13?g/dL, white blood cell count of 8,400/?L, and platelets of 380???109/L. Contrast-enhanced scan of chest, abdomen, and pelvis was performed and revealed no significant abnormality. The lesions were not associated with type B symptoms or bleeding manifestations ([Fig. 1A]). In the given clinicoradiological context, possibility of a soft tissue sarcoma and cutaneous lymphoma was considered.
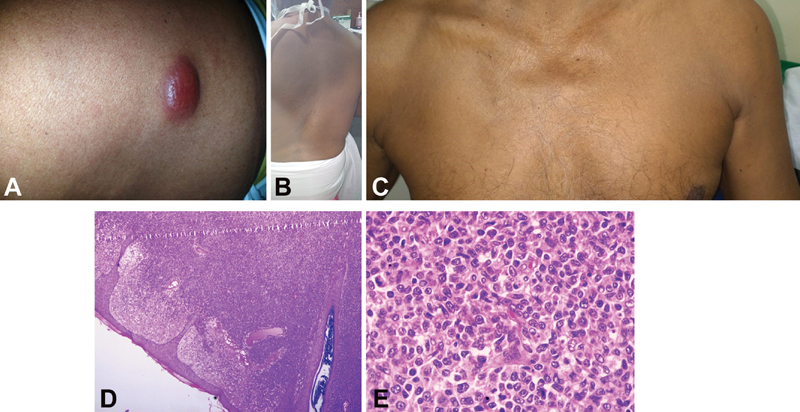
|?Fig. 1 (A)? Reddish painless nodules over the trunk; (B, C) Complete disappearance of all nodules by second week of therapy; (D, E) Hematoxylin and eosin?stained section show dermal tumor. The tumor cells are intermediate in size and show oval irregular nuclear contour, conspicuous nucleoli, and moderate cytoplasm. Brisk mitotic figures and apoptotic bodies noted (original magnification: 1d- ?200 and 1e- ?400).|
Complete excision biopsy of the nodule over the arm was done, and the blocks were processed and studied for histomorphology. Biopsy from the nodule revealed dermis infiltrated by atypical lymphoid cells arranged in diffuse sheets. Periadnexal and perivascular neoplastic cell aggregates were noted. The tumor cells were of intermediate size and showed oval nuclei, irregular nuclear contour, conspicuous nucleoli, and moderate eosinophilic cytoplasm. Prominent proliferating blood vessels were noted in the background. Frequent mitotic figures, atypical mitosis, and apoptotic bodies were identified. The tumor infiltrated the underlying subcutaneous tissue. Overlying epidermis was free of tumor ([Fig. 1D] and [E]).
On performing immunohistochemistry, the tumor diffusely expressed LCA (leukocyte common antigen) HLA DR (human leukocyte antigen - DR isotype), CD123, CD56, CD123, CD43, CD4, and CD68. Tumor cells were immunonegative for T-cell markers (CD3, CD8, and CD7), myeloid and monocytic cells (MPO, and CD117), B-cells (CD20), CK, CD10, BCL6, CD23, TIA, CD34, TdT, MUM1, TCR gamma and TCR Beta and EBV. TCL1 immunomarker was not performed due to unavailability. The MIB 1 labeling index was approximately 30% in areas of highest proliferative activity ([Fig. 2A?F]). Bone marrow biopsy did not reveal infiltration by abnormal cells. Thus, diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was made.
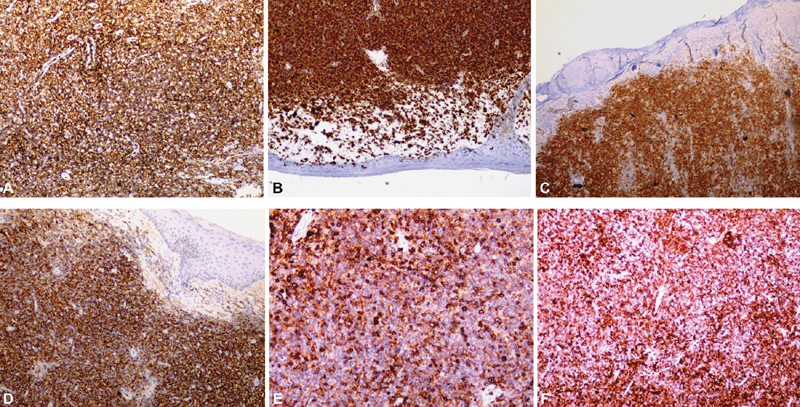
|?Fig. 2? The tumor cells expresses LCA (A), HLA-DR (B), CD123 (C), CD56 (D), CD43 (E), CD 68 (F) (original magnification: ?200).|
Considering the age of the patient, allogenic stem cell transplant (allo?stem cell transplantation [SCT]) was at consolidation. The patient was then initiated on a modified Berlin?Frankfurt?Munster (BFM) acute lymphoblastic leukemia (ALL)-like protocol with prednisolone, 60?mg/m2; weekly vincristine, 1.4?mg/m2; daunorubicin, 25?mg/m2; and L-asparaginase, 5,000 unit/m2?(2 doses a week as per institutional protocol adjusted to his age; total of 8 doses were delivered). He demonstrated a good response with complete disappearance of all nodules by second week of therapy ([Fig. 1B] and [C]) and is continuing the same modified BFM ALL-like protocol. The patient is being closely monitored and did not have any side effects like hyperglycemia, pancreatitis, or bleeding manifestation. Clinically, patient went into remission after third week induction with no new lesions and disappearance of nodules.
Discussion
BPDCN is a rare malignancy of dendritic cell precursors as it resembles the normal counterpart in expression profile, immunophenotype, and biological function.[5] [6] A study of its ontogeny shows the cell of origin to be much closer to myeloid precursors.[7] These are commonly called as natural killer (NK)-cell lymphoma, CD4?+?NK-cell leukemia, or blastic NK leukemia/lymphoma due to the expression of the CD56 molecule. The etiology of BPDCN is not clearly known except for its association with myelodysplastic syndromes (MDS) due to the cooccurrence of several gene mutations.[6] [7]
The disease commonly presents as asymptomatic cutaneous lesions.[7] [8] Extracutaneous involvement includes involvement of bone marrow and lymph nodes.[7] An isolated or disseminated bruise-like lesion has better outcome than more disseminated lesions.[9] [10] [11] [12] However, lack of initial cutaneous involvement has been reported in the literature like mucosal lesion in oral cavity.[13]
In cases of disseminated disease; biopsy of the cutaneous nodule, lymph node, or bone marrow biopsy is essential for establishment of the diagnosis. In cutaneous lesions, epidermis is usually spared, and the lesion typically infiltrates the dermis. As seen in our case, progression of disease often leads to involvement of subcutaneous fat. Monomorphic population of small to medium sized cells showing irregular nuclear contours, fine to evenly dispersed chromatin, small nucleoli, and scant to moderate amount of cytoplasm is characteristically seen in BPDCN.[14] [15] [16] [17] Plasmacytoid dendritic cells (PDCs) showed strong immunoexpression for CD4, CD123, and HLA-DR, while they focally show CD43 and CD68. Expression of CD123 (high) is the main characteristic of PDCs. When in doubt, immunomarker for TCL1 may be used to support the diagnosis of BPDCN. Plasmacytoid dendritic cells are the principal source of interferon ?, and their accumulation at inflammatory sites contributes to the immune and inflammatory response.[14] [15]
Plasmacytoid dendritic cells are predominantly seen in allergic rhinitis, hyaline vascular Castleman's disease, granulomatous lymphadenitis, Kikuchi?Fujimoto lymphadenopathy, and Hodgkin lymphoma. PDCs are also noticed in skin of patients with hydroa vacciniforme, lupus erythematosus, and psoriasis.[14] [15] In our case, the patient did not complain of any inflammatory lesion in the past. There is a strong debate that PDCs belong to the myelomonocytic lineage as AML - Acute Myeloid Leukemia and myelodysplasia can occur in the due course of this disease. World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissue (2008) had grouped BPDCN with the category of AML and related precursor neoplasms which now has been listed as its own category in the 2016 revised version.[18] [19]
BPDCN demonstrates deletion of several tumor suppressor genes including?CDKN2A,?RB1,?CDKN1B?and tumor protein p53 (TP53).?TET2?gene mutation (ten?eleven translocation?2), located on band 4q24, provides additional evidence that BPDCN is a myeloid related neoplasm.[16]
A biological hallmark of BPDCN is disruption at the G1/S transition that possibly contributes to pathogenesis.[16] Clinical features, morphologic findings, immunophenotypic profile, and cytogenetic and molecular data forms the basis for the diagnosis of BPDCN.[16] [17] [18] Among these, immunoprofile of neoplastic cells plays an important role in the diagnosis of the disease since there is an overlap of the immunophenotypic features with other hematopoietic neoplasms.[19]
Before establishing a diagnosis of BPDCN in particular, NK-cell lymphoma/leukemia, mature T-cell lymphomas/leukemias, T-cell lymphoblastic leukemia/lymphoma (T-ALL/LBL), and myeloid sarcoma/AML some should be excluded.[17] In our case, myeloid sarcoma was ruled out by immunonegativity of MPO and CD117; T-ALL/LBL was ruled out as the tumor cells were immunonegative for T-cell markers (CD3, CD8, and CD7); NK T-cell lymphoma/leukemia was ruled out as the tumor cells did not express T-cell nor cytotoxic markers.
BPDCN is inherently resistant to standard chemotherapies and have overall poor survival. Intensive ALL-like induction regimens (e.g., cyclophosphamide, vincristine, doxorubicin [also known by its trade name, Adriamycin], and dexamethasone hyper-CVAD - cyclophosphamide, vincristine, doxorubicin (also known by its trade name, Adriamycin), and dexamethasone; BFM chemotherapy) a were considered as more effective on comparison to standard therapies like CHOP - cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate (Oncovin), and prednisone.[4] [20] [21]
Combination of L-asparaginase and methotrexate has shown clinical activity in BPDCN, predominantly in elderly patients.[22] [23] Consolidation with allogeneic SCT (allo-SCT), in first CR - Complete response, demonstrated durable complete remissions with overall survival rates ranging from 58% at 3 years to 40% at 10 years. With regard to relapse rates, reduced intensity conditioning appears to be equivalent to myeloablative regimens.[24] Elderly patients are treated with lower intensity chemotherapy regimens like pralatrexate, bendamustine, or gemcitabine/docetaxel combinations or hypomethylating agents like 5-azacytidine which demonstrated transient responses.[25] [26] [27] [28]
Novel Agents in Blastic Plasmacytoid Dendritic Cell Neoplasm
Literature reveals that low-intensity treatments have unsatisfactory results, whereas intensive therapies lead to toxicity. Thus, there was a need for the use of novel targeted agents for the treatment of BPDCN.[29] SL-401 is a novel recombinant protein which includes components of diphtheria toxin fused with interleukin-3, demonstrating overall response rate of 77% (with 55% CR).[29] Promising result with CR rate of 79% (first line) and 31% CR rate, a phase-II study with SL401 has been shown and reported at the 2017 ASH - American Society of Hematology for relapsed/refractory patients.[27] [28] [29] Phase-I trials are ongoing with other immunotherapies targeting CD123 which includes immunoconjugates, chimeric antigen receptor (CAR)-T-cells, and bispecific antibodies.[28]
Venetoclax (BCL-2 inhibitor) has demonstrated high single-agent activity in myeloid malignancies. Currently, it is used in combination with induction chemotherapy. Bromodomain and extra terminal domain inhibitors (BETs) are being studied in preclinical tests.[27] [28]
SCT with myeloablative or reduced intensity regimen have shown improved survival in younger and selected elderly patients. The clinical course and response to the treatment is best when the procedure is performed after first CR28?30.
Conclusion
BPDCN is a very rare neoplasm with poor treatment outcomes. In younger patients, induction regimens (ALL-like) followed by allogenic SCT in CR-1 and in elderly unfit patients, L-asparaginase based chemotherapy or hypomethylating agents achieve good palliation. Novel therapies like anti-CD123-directed immunotherapies and venetoclax-based therapy have shown promise in clinical trials.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Statement
The authors hereby declare that Institutional Ethics Committee did not mandate IEC review for this study. The collection of data from electronic medical record (EMR) and laboratory reports do not require an informed consent form as per the hospital's standard operating procedures.
*?Authors have equally contributed for writing, review and editing the case report.
- Sapienza MR, Pileri A, Derenzini E. et al.?Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel) 2019; 11 (05) 595
- Facchetti F, Pileri SA, Agostinelli C. et al.?Cytoplasmic nucleophosmin is not detected in blastic plasmacytoid dendritic cell neoplasm. Haematologica 2009; 94 (02) 285-288
- Garnache-Ottou F, Feuillard J, Saas P.?Plasmacytoid dendritic cell leukaemia/lymphoma: towards a well defined entity?. Br J Haematol 2007; 136 (04) 539-548
- Pagano L, Valentini CG, Pulsoni A. et al; GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party).?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 2013; 98 (02) 239-246
- Swerdlow SH, Campo E, Harris NL. et al, eds.?WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours. Revised 4th ed. Vol 2.. Lyon, France: International Agency for Research on Cancer; 2017
- Sapienza MR, Abate F, Melle F. et al.?Blastic plasmacytoid dendritic cell neoplasm: genomics mark epigenetic dysregulation as a primary therapeutic target. Haematologica 2019; 104 (04) 729-737
- Sapienza MR, Fuligni F, Agostinelli C. et al; AIRC 5xMille consortium ?Genetics-driven targeted management of lymphoid malignancies and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm.?Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014; 28 (08) 1606-1616
- Demir?z AS, Demirkesen C, Saliho?lu A, T?z?ner N.?Blastic plasmacytoid dendritic cell neoplasia: a single center experience. Turk J Haematol 2020; 37 (01) 48-52
- Bekkenk MW, Jansen PM, Meijer CJLM, Willemze R.?CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol 2004; 15 (07) 1097-1108
- Suzuki R, Nakamura S, Suzumiya J. et al; NK-cell Tumor Study Group.?Blastic natural killer cell lymphoma/leukemia (CD56-positive blastic tumor): prognostication and categorization according to anatomic sites of involvement. Cancer 2005; 104 (05) 1022-1031
- Assaf C, Gellrich S, Whittaker S. et al.?CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. J Clin Pathol 2007; 60 (09) 981-989
- Cota C, Vale E, Viana I. et al.?Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol 2010; 34 (01) 75-87
- Rauh MJ, Rahman F, Good D. et al.?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: case series and literature review. Leuk Res 2012; 36 (01) 81-86
- Jegalian AG, Facchetti F, Jaffe ES.?Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol 2009; 16 (06) 392-404
- Petrella T, Facchetti F.?Tumoral aspects of plasmacytoid dendritic cells: what do we know in 2009?. Autoimmunity 2010; 43 (03) 210-214
- Lucioni M, Novara F, Fiandrino G. et al.?Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood 2011; 118 (17) 4591-4594
- Shi Y, Wang E.?Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med 2014; 138 (04) 564-569
- Swerdlow S, Campo E, Harris NL. et al.?WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed.. Lyon, France: IARC; 2008
- Arber DA, Orazi A, Hasserjian R. et al.?The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 (20) 2391-2405
- Deotare U, Yee KWL, Le LW. et al.?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-color flow cytometry diagnosis and hyperCVAD therapy. Am J Hematol 2016; 91 (03) 283-286
- Deotare U, Kim DD, Michelis FV, Lipton JH, Lipton JH.?Allogeneic hematopoietic stem cell transplantions in blastic plasmacytoid dendritic cell neoplasm in first complete remission: an effective therapy for a rare disease. Leuk Lymphoma 2016; 57 (08) 1942-1944
- Gruson B, Vaida I, Merlusca L. et al.?L-asparaginase with methotrexate and dexamethasone is an effective treatment combination in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol 2013; 163 (04) 543-545
- Gilis L, Lebras L, Bouafia-Sauvy F. et al.?Sequential combination of high dose methotrexate and L-asparaginase followed by allogeneic transplant: a first-line strategy for CD4+/CD56+ hematodermic neoplasm. Leuk Lymphoma 2012; 53 (08) 1633-1637
- Reimer P, R?diger T, Kraemer D. et al.?What is CD4+CD56+ malignancy and how should it be treated?. Bone Marrow Transplant 2003; 32 (07) 637-646
- Leitenberger JJ, Berthelot CN, Polder KD. et al.?CD4+ CD56+ hematodermic/plasmacytoid dendritic cell tumor with response to pralatrexate. J Am Acad Dermatol 2008; 58 (03) 480-484
- DiNardo CD, Pratz K, Pullarkat V. et al.?Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019; 133 (01) 7-17
- Ulrickson ML, Puri A, Lindstrom S, Cassaday RD, De Padova N, Becker PS.?Gemcitabine and docetaxel as a novel treatment regimen for blastic plasmacytoid dendritic cell neoplasm. Am J Hematol 2017; 92 (05) E75-E77
- Khwaja R, Daly A, Wong M, Mah? E, Cerquozzi S, Owen C.?Azacitidine in the treatment of blastic plasmacytoid dendritic cell neoplasm: a report of 3 cases. Leuk Lymphoma 2016; 57 (11) 2720-2722
- Frankel AE, Woo JH, Ahn C. et al.?Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014; 124 (03) 385-392
Address for correspondence
Publication History
Publication Date:
05 December 2021 (online)
? 2021. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1 (A)? Reddish painless nodules over the trunk; (B, C) Complete disappearance of all nodules by second week of therapy; (D, E) Hematoxylin and eosin?stained section show dermal tumor. The tumor cells are intermediate in size and show oval irregular nuclear contour, conspicuous nucleoli, and moderate cytoplasm. Brisk mitotic figures and apoptotic bodies noted (original magnification: 1d- ?200 and 1e- ?400).|

|?Fig. 2? The tumor cells expresses LCA (A), HLA-DR (B), CD123 (C), CD56 (D), CD43 (E), CD 68 (F) (original magnification: ?200).|
References
- Sapienza MR, Pileri A, Derenzini E. et al.?Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel) 2019; 11 (05) 595
- Facchetti F, Pileri SA, Agostinelli C. et al.?Cytoplasmic nucleophosmin is not detected in blastic plasmacytoid dendritic cell neoplasm. Haematologica 2009; 94 (02) 285-288
- Garnache-Ottou F, Feuillard J, Saas P.?Plasmacytoid dendritic cell leukaemia/lymphoma: towards a well defined entity?. Br J Haematol 2007; 136 (04) 539-548
- Pagano L, Valentini CG, Pulsoni A. et al; GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party).?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 2013; 98 (02) 239-246
- Swerdlow SH, Campo E, Harris NL. et al, eds.?WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours. Revised 4th ed. Vol 2.. Lyon, France: International Agency for Research on Cancer; 2017
- Sapienza MR, Abate F, Melle F. et al.?Blastic plasmacytoid dendritic cell neoplasm: genomics mark epigenetic dysregulation as a primary therapeutic target. Haematologica 2019; 104 (04) 729-737
- Sapienza MR, Fuligni F, Agostinelli C. et al; AIRC 5xMille consortium ?Genetics-driven targeted management of lymphoid malignancies and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm.?Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014; 28 (08) 1606-1616
- Demir?z AS, Demirkesen C, Saliho?lu A, T?z?ner N.?Blastic plasmacytoid dendritic cell neoplasia: a single center experience. Turk J Haematol 2020; 37 (01) 48-52
- Bekkenk MW, Jansen PM, Meijer CJLM, Willemze R.?CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol 2004; 15 (07) 1097-1108
- Suzuki R, Nakamura S, Suzumiya J. et al; NK-cell Tumor Study Group.?Blastic natural killer cell lymphoma/leukemia (CD56-positive blastic tumor): prognostication and categorization according to anatomic sites of involvement. Cancer 2005; 104 (05) 1022-1031
- Assaf C, Gellrich S, Whittaker S. et al.?CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. J Clin Pathol 2007; 60 (09) 981-989
- Cota C, Vale E, Viana I. et al.?Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol 2010; 34 (01) 75-87
- Rauh MJ, Rahman F, Good D. et al.?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: case series and literature review. Leuk Res 2012; 36 (01) 81-86
- Jegalian AG, Facchetti F, Jaffe ES.?Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol 2009; 16 (06) 392-404
- Petrella T, Facchetti F.?Tumoral aspects of plasmacytoid dendritic cells: what do we know in 2009?. Autoimmunity 2010; 43 (03) 210-214
- Lucioni M, Novara F, Fiandrino G. et al.?Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood 2011; 118 (17) 4591-4594
- Shi Y, Wang E.?Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med 2014; 138 (04) 564-569
- Swerdlow S, Campo E, Harris NL. et al.?WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed.. Lyon, France: IARC; 2008
- Arber DA, Orazi A, Hasserjian R. et al.?The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 (20) 2391-2405
- Deotare U, Yee KWL, Le LW. et al.?Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-color flow cytometry diagnosis and hyperCVAD therapy. Am J Hematol 2016; 91 (03) 283-286
- Deotare U, Kim DD, Michelis FV, Lipton JH, Lipton JH.?Allogeneic hematopoietic stem cell transplantions in blastic plasmacytoid dendritic cell neoplasm in first complete remission: an effective therapy for a rare disease. Leuk Lymphoma 2016; 57 (08) 1942-1944
- Gruson B, Vaida I, Merlusca L. et al.?L-asparaginase with methotrexate and dexamethasone is an effective treatment combination in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol 2013; 163 (04) 543-545
- Gilis L, Lebras L, Bouafia-Sauvy F. et al.?Sequential combination of high dose methotrexate and L-asparaginase followed by allogeneic transplant: a first-line strategy for CD4+/CD56+ hematodermic neoplasm. Leuk Lymphoma 2012; 53 (08) 1633-1637
- Reimer P, R?diger T, Kraemer D. et al.?What is CD4+CD56+ malignancy and how should it be treated?. Bone Marrow Transplant 2003; 32 (07) 637-646
- Leitenberger JJ, Berthelot CN, Polder KD. et al.?CD4+ CD56+ hematodermic/plasmacytoid dendritic cell tumor with response to pralatrexate. J Am Acad Dermatol 2008; 58 (03) 480-484
- DiNardo CD, Pratz K, Pullarkat V. et al.?Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019; 133 (01) 7-17
- Ulrickson ML, Puri A, Lindstrom S, Cassaday RD, De Padova N, Becker PS.?Gemcitabine and docetaxel as a novel treatment regimen for blastic plasmacytoid dendritic cell neoplasm. Am J Hematol 2017; 92 (05) E75-E77
- Khwaja R, Daly A, Wong M, Mah? E, Cerquozzi S, Owen C.?Azacitidine in the treatment of blastic plasmacytoid dendritic cell neoplasm: a report of 3 cases. Leuk Lymphoma 2016; 57 (11) 2720-2722
- Frankel AE, Woo JH, Ahn C. et al.?Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014; 124 (03) 385-392


 PDF
PDF  Views
Views  Share
Share

