Bone Marrow Infiltration by Nonhematopoetic Small Round Cell Tumors: A Clinicopathological Study from a Tertiary Care Centre in South India
CC BY-NC-ND 4.0 Indian J Med Paediatr Oncol 2019; 40(S 01): S1-S5
DOI: DOI: 10.4103/ijmpo.ijmpo_218_17
Abstract
Objectives: The objective of this study to comprehensively analyze bone marrow (BM) infiltration by nonhematological round cell tumors. Materials and Methods: A total of 206 diagnosed cases of small round blue cell tumors (excluding lymphomas) during a period of 2 years, referred for BM examination were included in the study. Clinical details were obtained from medical records. BM aspiration (BMA) and BM biopsies (BMBx) were performed under local anesthesia for staging workup. BMBx were studied for cellularity, presence of infiltration by round cells (nonhematopoietic), histologic patterns (island/nests and diffuse sheets), fibrosis, necrosis and other secondary changes. Immunohistochemistry panel was used depending on the morphology. Results: The cases included age range from 45 days to 25 years with a median age of 12 years. There was a male predominance with male:female 1.5:1. Among these, 37/206 cases (17.9%) were positive for BM involvement (BMI) on BMBx. Of these, 24 cases were neuroblastoma (64.8%), 9 cases Ewing s sarcoma/primitive neuroectodermal tumor (24.3%), and 4 Rhabdomyosarcoma (10.8). BMBx was done in all the 206 cases. Among these, 37/206 cases were positive for BMI on BMBx while 35/206 cases were positive on BM imprints and 33/206 cases were positive on BMA. Conclusion: Detection of metastasis in the BM has both therapeutic and prognostic significance. BMBx are complementary in the diagnosis of small round cell tumor.
Keywords
Bone marrow biopsy - Ewings/primitive neuroectodermal tumor - marrow infiltration - neuroblastoma - nonhematopoetic small round cell tumorsPublication History
Publication Date:
24 May 2021 (online)
2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objectives: The objective of this study to comprehensively analyze bone marrow (BM) infiltration by nonhematological round cell tumors. Materials and Methods: A total of 206 diagnosed cases of small round blue cell tumors (excluding lymphomas) during a period of 2 years, referred for BM examination were included in the study. Clinical details were obtained from medical records. BM aspiration (BMA) and BM biopsies (BMBx) were performed under local anesthesia for staging workup. BMBx were studied for cellularity, presence of infiltration by round cells (nonhematopoietic), histologic patterns (island/nests and diffuse sheets), fibrosis, necrosis and other secondary changes. Immunohistochemistry panel was used depending on the morphology. Results: The cases included age range from 45 days to 25 years with a median age of 12 years. There was a male predominance with male:female 1.5:1. Among these, 37/206 cases (17.9%) were positive for BM involvement (BMI) on BMBx. Of these, 24 cases were neuroblastoma (64.8%), 9 cases Ewing s sarcoma/primitive neuroectodermal tumor (24.3%), and 4 Rhabdomyosarcoma (10.8). BMBx was done in all the 206 cases. Among these, 37/206 cases were positive for BMI on BMBx while 35/206 cases were positive on BM imprints and 33/206 cases were positive on BMA. Conclusion: Detection of metastasis in the BM has both therapeutic and prognostic significance. BMBx are complementary in the diagnosis of small round cell tumor.
Keywords
Bone marrow biopsy - Ewings/primitive neuroectodermal tumor - marrow infiltration - neuroblastoma - nonhematopoetic small round cell tumorsIntroduction
Malignant small round cell tumors is a term used for tumors composed of malignant round cells that are slightly larger or double the size of red blood cells (RBCs) in air-dried smears and patternless sheets of small round cells with dense cellularity and high N:C ratio on hematoxylin and eosin (H and E). Bone marrow (BM) is commonly infiltrated by hematopoietic tumors. Metastasis of nonhematopoietic tumor cells to BM was first reported in 1834; however, a series of such cases was not published until 1936.[1] In 1958, McFarland and Dameshek [2] described a simplified technique for BM biopsy and demonstrated that one could discover unsuspected malignant disease and in many cases confirm the finding obtained by aspiration. The extension of a cancer is a major prognostic factor (Stage IV disease) which determines the therapeutic strategy.
Small round cell tumors present more commonly during childhood and adolescent. Nonhematopoietic small round cell tumors include: Ewing sarcoma/primitive neuroectodermal tumor, retinoblastoma, rhabdomyosarcoma, nephroblastoma, mesenchymal chondrosarcoma, small cell osteosarcoma, poorly differentiated chordoma, melanotic neuroectodermal tumor, desmoplastic small round cell tumor, and germ cell tumors.[3] The recognition of metastasis in random biopsies presents challenges to pathologists when diagnosing the primary focus and needs an extensive workup.[4]
The recent improvements in immunohistochemistry and molecular biology methods enable to detect tumor cells in various sites such as lymph nodes, BM, and blood with a considerably increased sensitivity as compared to conventional approaches.[5] This study was undertaken to comprehensively analyze BM metastasis of nonhematological round cell tumors diagnosed at a single tertiary care center in South India over the 2 years.
Materials and Methods
This retrospective study was conducted from January 2014 to June 2016. A total of 206 diagnosed cases of small round blue cell tumors (excluding lymphomas), referred for BM examination were included in the study. Age and sex distribution, clinical findings and investigations (including ultrasound, X-rays, computed tomography scan, magnetic resonance imaging, and biopsy findings of primary tumor) of all patients were noted. An ethylenediaminetetraacetic acid-blood sample was obtained for complete blood picture and peripheral blood film examination. Peripheral blood smears were stained by Leishmann stain. BM aspiration (BMA) was performed by 16G lumbar puncture needle and subjected to staining by Giemsa stain. BM biopsies (BMBx) were obtained using the conventional technique with a Jamshidi needle from the posterior superior iliac spine under local anesthesia. The biopsies were of adequate (1.5 2 cm) length and fixed in 10% formalin solution and decalcified using 10% formal formic acid for 4 6 h followed by routine processing and paraffin embedding.[5] Serial sections of 4 6 m thickness were cut and stained by H and E and supplemented with immune stains where ever required. Hematological parameters including hemoglobin, total leukocyte count and platelet count, and peripheral blood film examination with RBC morphology or presence of any atypical cells on peripheral blood were noted. A detailed examination of BM smears was done particularly for presence of atypical cells. BMBx sections with at least five well preserved marrow spaces were studied for cellularity, normal hematological elements, presence of infiltration by round cells (nonhematopoietic), histologic pattern and morphology of infiltration (island/nests, diffuse sheets), reticulin fibrosis, necrosis, and other secondary changes. Immunohistochemistry (IHC) panel included CD56, CD99, synaptophysin, chromogranin, S100, desmin, myogenin, and vimentin depending on the morphology.
Results
Demographic and clinical aspects
BMA (n = 206), imprints BMA, and BMBx (n = 206) were studied for involvement by round cell tumors from January 2014 to June 2016. The cases included age range from 45 days to 25 years with a median age of 12 years. There was a male predominance with male: female 1.5:1.
Bone marrow aspirate and biopsy findings
A total number of BMA were 4137 of which 206 (5%) had an established clinical diagnosis of round cell tumor. Among these, 33/206 (16.01%) cases were positive for BM involvement (BMI). Erythroid hyperplasia was seen in 10/206 (4.8%), reactive marrow in 10/206 (4.8%) and marrow within normal limits in 186/206 (90.2%). Leukoerythroblastic picture was seen in 9/206 (4.3%) cases on peripheral blood examination.
In 33 cases, BMA showed involvement by round cell tumor, in two cases, tumor was picked up in the imprint smear and biopsy, while in two other cases, both aspirate and imprint smears were negative but was positive on biopsy. Of the 33 positive cases in BMA, 22 cases were neuroblastoma (66.6%), 8 cases Ewing s sarcoma (24.2%), and 3 cases of rhabdomyosarcoma (9.1%). Distribution of the cases with BMI and IHC is shown in [Table 1].
|
Type of SRCT |
Clinical Dx of SRCT (n=206) |
Number of cases with BMA involvement (n=33/206) |
Number of cases with BM imprint involvememt (n=35/206) |
Number of cases with BMBx involvement (n=37/206) |
|---|---|---|---|---|
|
BM Bone marrow; BMBx BM biopsy; SRCT Small round cell tumors; BMA BM aspiration; PNET Primitive neuroectodermal tumor; BMI BM involvement |
||||
|
Neuroblastoma |
84 |
22 |
22 |
24 (including 2 cases that were -ve on BMA and BMI) |
|
Ewings sarcoma/PNET |
80 |
8 |
9 (including 1 case that was ve on BMA) |
9 (including 1 case that was ve on BMA) |
|
Rhabdomyosarcoma |
35 |
3 |
4 (including 1 case that was ve on BMA) |
4 (including 1 case that was ve on BMA) |
|
Medulloblastoma |
4 |
- |
- |
- |
|
Wilms tumor |
2 |
- |
- |
- |
|
Hepatoblastoma |
1 |
- |
- |
- |
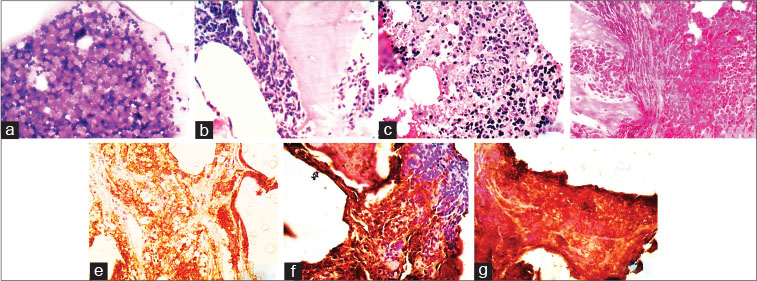
| Fig. 1 A case of neuroblastoma with bone marrow infiltratio (a) trephine imprint showing atypical round cells ( 400) (b d) Bone marrow biopsy displaying paratrabecular, interstitial and diffuse pattern of infiltratio by round cells, respectively ( 100) (e) immunohistochemistry with CD56 showing cytoplasmic and membranous positivity ( 100), (f and g) the round cells showing positivity for vimentin and neuron specifi enolase, respectively ( 100)
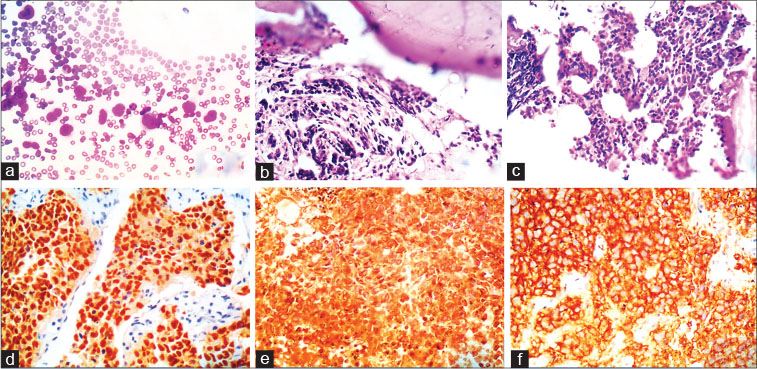
| Fig. 2 A case of rhabdomyosarcoma with bone marrow infiltratio (a) bone marrow aspiration smears showing singly scattered large atypical round cells with scant cytoplasm and large bizarre nuclei with dispersed chromatin ( 400) (b and c) bone marrow biopsy displaying paratrabecular and interstitial pattern of infiltratio by round cells, respectively ( 400 and 100). (d f) immunohistochemistry with myogenin, vimentin, and CD56 showing positivity ( 100)
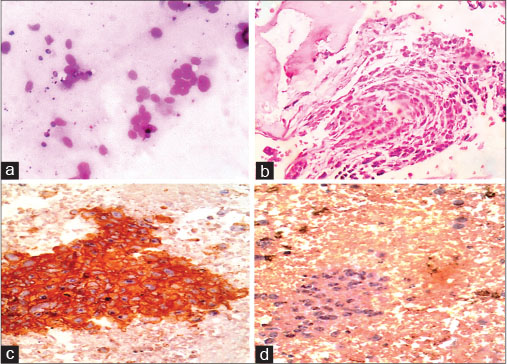
| Fig. 3 Figure 3: A case of Ewings/primitive neuroectodermal tumor with bone marrow infiltratio (a) bone marrow aspiration smears showing rosettes and singly scattered round cells with scant cytoplasm and round nuclei with dispersed chromatin ( 400) (b) bone marrow biopsy displaying paratrabecular pattern of infiltration by round cells ( 400) (c and d) immunohistochemistry with CD99 showing membrane and cytoplasmic positivity and negativity with CD56 ( 400)
In our study, 33 cases showed involvement by round cell tumor on BMA, in 2 cases tumor was picked up in the imprint smear and biopsy, while in 2 other cases both aspirate and imprint smears were negative; however, biopsy was positive, with an overall positive cases being 37 of the total 206 cases of small round cell tumors.
There have been several previous studies showing superiority of the BMBx over aspirate smears in diagnosis of metastatic tumor [12] [13] as deposits in the marrow are focal and may often elicit a fibrotic response, and therefore, aspirates may be negative. Multiple sections of the biopsy enable a much larger volume of the marrow to be examined and allow infiltration to be recognized.[14] Singh et al.[12] reported that BMBx was superior to aspiration (97% vs. 72%), Mishra et al. showed (100% vs. 91.3%) similar findings were observed in our study where biopsy versus aspiration positivity is (100% vs. 89.1%) [Table 2]. However, a study conducted by Sharma et al.[15] showed an occasional case where metastasis was detected only in aspiration. Bearden et al.[16] reported that BMAs and BMBx were complementary in diagnosis of various solid tumors.
|
Features |
Present study (n=206), n (%) |
Naghmi Asif et al. (n=82), n (%) |
Mishra et al. (n=50), n (%) |
Rafiq et al. (n=14), n (%) |
|---|---|---|---|---|
|
BM Bone marrow; BMBx BM biopsy; BMA BM aspiration |
||||
|
Age range |
1.5 months to 25 years |
6 months to 22 years |
1-65 years |
1-25 years |
|
BMA involvement by small round cell tumor |
33/206 (16.01) |
16/82 (19.5) |
9/50 (18) |
7/14 (50) |
|
BMBx involvement by small round cell tumor |
37/206 (18) |
14 (17) |
9/50 (18) |
7/14 (50) |
|
Neuroblastoma |
24/84 (28.5) |
2/5 (40) |
2/5 (40) |
3/3 (100) |
|
Ewing's sarcoma |
9/80 (11.2) |
3/6 (50) |
6/28 (21) |
1/2 (50) |
|
Rhabdomyosarcoma |
3/35 (8) |
3/8 (37) |
0/9 (0) |
2/3 (66) |
- Samson M. Fait remarkable de diathesecancereuse. Gaz Med Paris 1834; 2: 140
- Rohr K, Hegglin R. Tumorzellen in sternapunktat. Dtsch Arch Klin Med 1936; 179: 61-79
- Asif N, Hassan K, Yasmeen N. Bone marrow infiltration in small blue cell tumours. J Islamabad Med Dent Coll (JIMDC) 2013; 2: 3-8
- Papac RJ. Bone marrow metastases. A review. Cancer 1994; 74: 2403-13
- Schleiermacher G, Delattre O. Detection of micrometastases and circulating tumour cells using molecular biology technics in solid tumours. Bull Cancer 2001; 88: 561-70
- Contreras E, Ellis LD, Lee RE. Value of the bone marrow biopsy in the diagnosis of metastatic carcinoma. Cancer 1972; 29: 778-83
- Leland J, MacPherson B. Hematologic findings in cases of mammary cancer metastatic to bone marrow. Am J Clin Pathol 1979; 71: 31-5
- Rubins JM. The role of myelofibrosis in malignant leukoerythroblastosis. Cancer 1983; 51: 308-11
- Mishra P, Das S, Kar R, Jacob SE, Basu D. Non-haematopoietic malignancies metastasing to the bone marrow: A 5 year record-based descriptive study from a tertiary care centre in South India. Indian J Cancer 2014; 51: 30-4
- Rafiq H, Khurshid R, Zia R, Hayee A. Frequency of bone invasion and metastasis in round cell tumors of pediatric age groups. Biomedica 2007; 23: 8-11
- Kumar L, Majhi U, Shanta V. Frequency of bone marrow involvement in non-haematological malignancies. J Assoc Physicians India 1990; 38: 553-5
- Singh G, Krause JR, Breitfeld V. Bone marrow examination: For metastatic tumor: Aspirate and biopsy. Cancer 1977; 40: 2317-21
- Ingle JN, Tormey DC, Tan HK. The bone marrow examination in breast cancer: Diagnostic considerations and clinical usefulness. Cancer 1978; 41: 670-4
- Basu D, Singh T, Shinghal RN. Micrometastasis in bone marrow in breast cancer. Indian J Pathol Microbiol 1994; 37: 159-64
- Sharma S, Murari M. Bone marrow involvement by metastatic solid tumors. Indian J Pathol Microbiol 2003; 46: 382-4
- Bearden JD, Ratkin GA, Coltman CA. Comparison of the diagnostic value of bone marrow biopsy and bone marrow aspiration in neoplastic disease. J Clin Pathol 1974; 27: 738-40
- Imran R, Aejaz S, Banday MA, Ahmad SN. Extrarenal Wilms tumour with bone marrow involvement: An index case report. Chin Ger J Clin Oncol 2010; 9: 295-7
- Krskov L, Mrhalov M, Hilsk I, Sumerauer D, Drahokoupilov E, M dry P. et al. Detection and clinical significance of bone marrow involvement in patients with rhabdomyosarcoma. Virchows Arch 2010; 456: 463-72
Address for correspondence
Publication History
Publication Date:
24 May 2021 (online)
2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig. 1 A case of neuroblastoma with bone marrow infiltratio (a) trephine imprint showing atypical round cells ( 400) (b d) Bone marrow biopsy displaying paratrabecular, interstitial and diffuse pattern of infiltratio by round cells, respectively ( 100) (e) immunohistochemistry with CD56 showing cytoplasmic and membranous positivity ( 100), (f and g) the round cells showing positivity for vimentin and neuron specifi enolase, respectively ( 100)

| Fig. 2 A case of rhabdomyosarcoma with bone marrow infiltratio (a) bone marrow aspiration smears showing singly scattered large atypical round cells with scant cytoplasm and large bizarre nuclei with dispersed chromatin ( 400) (b and c) bone marrow biopsy displaying paratrabecular and interstitial pattern of infiltratio by round cells, respectively ( 400 and 100). (d f) immunohistochemistry with myogenin, vimentin, and CD56 showing positivity ( 100)

| Fig. 3 Figure 3: A case of Ewings/primitive neuroectodermal tumor with bone marrow infiltratio (a) bone marrow aspiration smears showing rosettes and singly scattered round cells with scant cytoplasm and round nuclei with dispersed chromatin ( 400) (b) bone marrow biopsy displaying paratrabecular pattern of infiltration by round cells ( 400) (c and d) immunohistochemistry with CD99 showing membrane and cytoplasmic positivity and negativity with CD56 ( 400)
References
- Samson M. Fait remarkable de diathesecancereuse. Gaz Med Paris 1834; 2: 140
- Rohr K, Hegglin R. Tumorzellen in sternapunktat. Dtsch Arch Klin Med 1936; 179: 61-79
- Asif N, Hassan K, Yasmeen N. Bone marrow infiltration in small blue cell tumours. J Islamabad Med Dent Coll (JIMDC) 2013; 2: 3-8
- Papac RJ. Bone marrow metastases. A review. Cancer 1994; 74: 2403-13
- Schleiermacher G, Delattre O. Detection of micrometastases and circulating tumour cells using molecular biology technics in solid tumours. Bull Cancer 2001; 88: 561-70
- Contreras E, Ellis LD, Lee RE. Value of the bone marrow biopsy in the diagnosis of metastatic carcinoma. Cancer 1972; 29: 778-83
- Leland J, MacPherson B. Hematologic findings in cases of mammary cancer metastatic to bone marrow. Am J Clin Pathol 1979; 71: 31-5
- Rubins JM. The role of myelofibrosis in malignant leukoerythroblastosis. Cancer 1983; 51: 308-11
- Mishra P, Das S, Kar R, Jacob SE, Basu D. Non-haematopoietic malignancies metastasing to the bone marrow: A 5 year record-based descriptive study from a tertiary care centre in South India. Indian J Cancer 2014; 51: 30-4
- Rafiq H, Khurshid R, Zia R, Hayee A. Frequency of bone invasion and metastasis in round cell tumors of pediatric age groups. Biomedica 2007; 23: 8-11
- Kumar L, Majhi U, Shanta V. Frequency of bone marrow involvement in non-haematological malignancies. J Assoc Physicians India 1990; 38: 553-5
- Singh G, Krause JR, Breitfeld V. Bone marrow examination: For metastatic tumor: Aspirate and biopsy. Cancer 1977; 40: 2317-21
- Ingle JN, Tormey DC, Tan HK. The bone marrow examination in breast cancer: Diagnostic considerations and clinical usefulness. Cancer 1978; 41: 670-4
- Basu D, Singh T, Shinghal RN. Micrometastasis in bone marrow in breast cancer. Indian J Pathol Microbiol 1994; 37: 159-64
- Sharma S, Murari M. Bone marrow involvement by metastatic solid tumors. Indian J Pathol Microbiol 2003; 46: 382-4
- Bearden JD, Ratkin GA, Coltman CA. Comparison of the diagnostic value of bone marrow biopsy and bone marrow aspiration in neoplastic disease. J Clin Pathol 1974; 27: 738-40
- Imran R, Aejaz S, Banday MA, Ahmad SN. Extrarenal Wilms tumour with bone marrow involvement: An index case report. Chin Ger J Clin Oncol 2010; 9: 295-7
- Krskov L, Mrhalov M, Hilsk I, Sumerauer D, Drahokoupilov E, M dry P. et al. Detection and clinical significance of bone marrow involvement in patients with rhabdomyosarcoma. Virchows Arch 2010; 456: 463-72


 PDF
PDF  Views
Views  Share
Share

