Breast Imaging: Past and Present
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(01): 109-113
DOI: DOI: 10.1055/s-0042-1742641
Abstract
Breast imaging has evolved over several decades; however, a significant leap has happened in the past couple of decades. Multiple modalities are available for the evaluation of breast diseases; Mammography, Digital Breast Tomosynthesis, Ultrasound, Magnetic Resonance Imaging (MRI), and Contrast Enhanced Mammography (CEM) are a few of the frequently used ones. Image-guided interventions have further evolved, core needle biopsy, vacuum-assisted biopsy, clip placements, and wire localization to name a few. At times, it is difficult to choose between the modalities for disease evaluation and intervention. In this article, we have tried to cover in brief the evolution of breast imaging over years and have discussed the imaging approach to some frequent clinical presentations along with the approach to the evaluation of mammography.
Publication History
Article published online:
15 February 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Breast imaging has evolved over several decades; however, a significant leap has happened in the past couple of decades. Multiple modalities are available for the evaluation of breast diseases; Mammography, Digital Breast Tomosynthesis, Ultrasound, Magnetic Resonance Imaging (MRI), and Contrast Enhanced Mammography (CEM) are a few of the frequently used ones. Image-guided interventions have further evolved, core needle biopsy, vacuum-assisted biopsy, clip placements, and wire localization to name a few. At times, it is difficult to choose between the modalities for disease evaluation and intervention. In this article, we have tried to cover in brief the evolution of breast imaging over years and have discussed the imaging approach to some frequent clinical presentations along with the approach to the evaluation of mammography.
Attempts to evaluate breasts using X-rays started way back in the 1920s. Those initial attempts were not rewarding as the images generated were of poor quality.[1]
In 1930, the first study of 119 patients evaluated using X-rays was published by Stafford Warren, MD, with an accuracy of 93%. However, those images were also of poor quality. The next big change happened after a long gap of about 30 years, around 1960, with Egan's description of a standardized direct-exposure mammographic technique and a report on 1,000 breast cancer cases.[2] This technique had a ceiling-mounted X-ray tube and a special generator to produce lower peak kilovoltage X-rays. In 1970, there was a brief spark with the development of xeroradiography; however, it did not last long as it involved high-radiation exposure and inconsistent results. Then, started the era of screen-film mammography.[3] At around the same time, a uniform compression technique was developed. This was a good time in the development of breast imaging, as two breakthroughs happened around the same time.
The screen-film mammography technique nearly completely replaced direct exposure mammography. It has faster image acquisition time and less radiation exposure. With the uniform compression technique, there was a reduction in motion blur and a further decrease in radiation dose.[3]
With continuous improvement in the screen-film technique, there was continued improvement in image quality.
Health insurance policy (HIP) trial needs a separate mention here, results of this trial led to a formal breast screening program. This trial was started in 1963 to explore the efficacy of screening. Women aged 40 to 64 years were selected to enroll in the HIP of Greater New York and were randomly assigned to study and control groups. After 18 years, the trial was concluded. The results showed 25% reduction in mortality from breast cancer among women who were screened in both age groups of 40 to 49 and 50 to 59.[4]
Now is the era of digital mammography, also described as full-field digital mammography (FFDM). In this technique, digital receptors are used in the place of screen film. There are manifold advantages of this technique. Digital images are incorporated with the picture archiving and communication system. They are easy to reproduce without exposing patients again to radiation, post-processing of image is possible, they can be stored for a long time without losing their quality, and can be used for comparison at a later time ([Fig. 1A] and [B]).
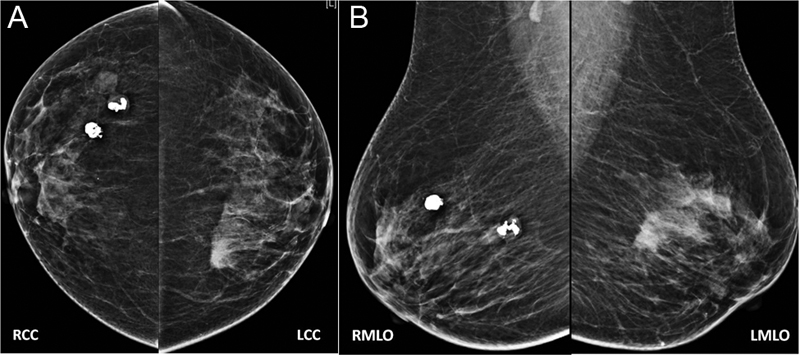
| Fig. 1(A and B) With Illustration 1: bilateral mammogram in CC and MLO views. It shows two popcorn calcifications in the upper outer quadrants of the right breast suggestive of involuting fibroadenomas. A high-density mass in the upper inner quadrant of the left breast is seen.
The latest development in breast imaging is the three-dimensional (3D) mammography technique, also known as digital breast tomosynthesis (DBT). DBT got Food and Drug Administration approval in the year 2011 for use in breast imaging.[5]
This technique involves obtaining multiple thin images of breasts in a stack. This is obtained by movement of the X-ray tube in arc-like fashion for 15° to 60° depending on the vendor. Each image is evaluated as a separate image in a stack. DBT was used along with 2D FFDM; however, it raised concerns over increasing radiation dose to patients. This has led to the development of synthesized views, which are constructed from data of DBT. Since its introduction, there has been a significant change in the practice of breast imaging. This technique has led to an improved cancer detection rate and a decrease in the recall rate.[6]
Data from a study suggested that DBT is associated with the detection of smaller, more often node-negative and HER2-negative invasive cancer when compared with 2D FFDM.[7]
A recent entry in breast imaging is contrast-enhanced mammogram (CEM).[8]
Iodinated contrast is introduced intravenously, and subsequently, low and high-energy X-ray images are obtained. These images are then post-processed to get subtracted images. The post-processed images show only contrast enhancement within the breast, masking background parenchymal density ([Fig. 2]). This new technique has many promising advantages: it is cheap, fast, and many existing mammography machines can be upgraded to incorporate this technique. It is especially useful in locations where access to breast magnetic resonance imaging (MRI) is limited. This technique is also described as poor women's MRI. However, being a fairly recent technology, many studies are occurring around the world for its use in different indications.
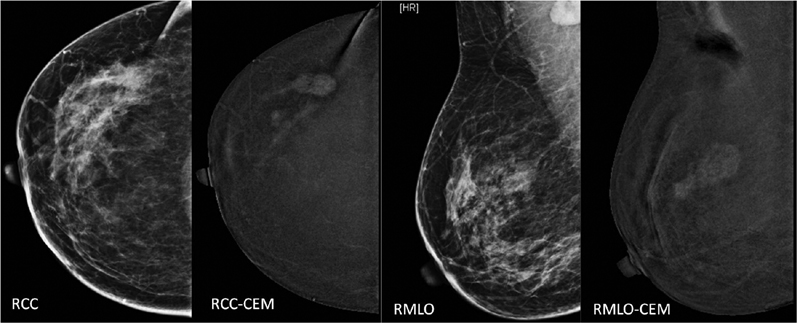
| Fig.2:Mammogram (= low-density image on CEM) and processed contrast-enhanced images of the right breast in CC and MLO views, showing enhancement of lesion in the upper outer quadrant with suppression of background parenchymal density. Adjacent satellite lesion is better seen on contrast image (CEM) on CC view. CC, craniocaudal; CEM, contrast-enhanced mammogram; MLO; mediolateral oblique.
Mammography and ultrasound (US) are complementary to each other. Since US has been introduced as a diagnostic imaging investigation in the year 1954, it has been an integral part of the investigation of breast diseases.[9]
Many advances have happened in the US technique and equipment: harmonic imaging, cross-beam technology, elastography, etc. One of the important ones is elastography which measures the stiffness of tissue with an US beam. The harder the tissue, the more are the chances of it being malignant. The hardness of tissue can also be measured quantitatively in kilopascal. Elastography can be performed either with shear wave elastography or strain elastography. Shear wave elastography can quantitatively measure stiffness in kilopascal or meters per second.[10] Initial attempts for breast MRIs were performed using body coils. Subsequently, breast coils were developed for dedicated breast imaging. MRI has evolved on two fronts: spatial imaging and dynamic imaging with kinetic curve assessment. With improvement in spatial imaging techniques, high-quality images are obtained, whereas dynamic images give information about vascularity and angiogenesis within the tissue. Kinetic curve assessment has shown a good predictive value for cancer detection. There are various indications when breast MRI is performed: a surveillance scan in BRCA-positive cases, in high-risk individuals, as a problem-solving tool when both mammogram and US are inconclusive, in cases of lobular cancer on histopathology for disease mapping, in the evaluation of nipple discharge when US is negative, and response assessment in post-neoadjuvant chemotherapy (NACT) cases.
MRI has high sensitivity in the detection of small lesions; however, it is still not the first imaging investigation in breast cancer evaluation, for multiple reasons. First of all, it is expensive and time-consuming; MRI is not sensitive for the detection of calcifications, which is one of the common presentations of early noninvasive cancer. At times, calcification seen on mammogram due to low-grade ductal carcinoma in situ (DCIS) are completely silent on MRI, without any enhancement. Hence, performing only an MRI is insufficient, as it might miss small grouped microcalcifications in some other parts of the breast. MRI is used as an adjunct to mammogram and US.
Various image-guided breast interventions can be performed. These are either diagnostic or therapeutic. Image-guided fine needle aspirations, biopsies, and clip placements have changed the way of management of breast diseases over the years. Image-guided wire localizations have improvized the management of smaller lesions to less extensive surgical procedures. Image-guided clip placement pre-NACT enables breast conservative surgery in selected larger lesions. Therapeutic image-guided interventions are aspiration of cysts or abscesses, vacuum-assisted excisions of fibroadenomas, or other B3 lesions. The image-guided cryoablation of breast cancer is under initial studies for therapeutic use.
Image guidance for breast intervention procedures is performed using US, stereotactic, or MRI guidance. The dictum is to use the modality which shows the best pathology. There is a general trend toward having a re-examination of US (re-look US) for lesions seen on MRI/mammography, as US-guided interventions are easy to perform, with real-time imaging of lesions during the procedure. However, this may not apply to all pathologies. Microcalcifications which are only seen on mammograms are to be addressed with stereotactic or tomosynthesis guidance, whereas non-mass enhancement is only seen on MRI and needs MRI-guided intervention.
Approach to Imaging of Breast Disease
Many developed countries around the world have dedicated mammography screening protocols. There are a few differences in these programs: starting age of screening and the time gap between two screening examinations. But all programs are based on the fact that screening is useful in early cancer detection and reduction in mortality. Screening mammograms are performed for asymptomatic individuals, whereas diagnostic imaging is performed for symptomatic patients.
American College of Radiology (ACR) and Society of Breast Imaging recommend annual screening mammogram starting at 40 years of age. The National Health Service (UK) starts screening at 50 years of age and mammogram performed after every 3-year interval. Australia and Singapore invite women for screening at the age of 50 years to have a 2-yearly mammogram. In India, we do not have a screening program for the general population. Opportunistic screening is performed whenever feasible. However, high-risk cases like BRCA mutation positive, strong family history can be called for screening annually. BRCA-positive cases are screened with mammogram and MRI alternately every 6 months.
Now, we will discuss approach to diagnostic imaging in a few clinical cases and, in the end, a brief approach to the interpretation of mammograms.
Clinical Scenarios
Case 1
A 55-year-old lady complains of a breast lump.
The investigation of choice is mammography. Mammography helps in the detection of breast mass along with calcifications. Various parameters about mass like shape, density, margin, any other suspicious satellite lesions, and associated features like architectural distortion, adjacent skin changes, and microcalcifications are assessed with mammograms ([Fig. 3]). At times microcalcifications extend beyond the mass, and in such cases the true extent of disease is larger than the mass or palpable abnormality seen on mammogram. Once the mammogram is evaluated, mass is further evaluated on US and appropriate breast imaging-reporting and data system (BI-RADS) category is assigned to the mass.
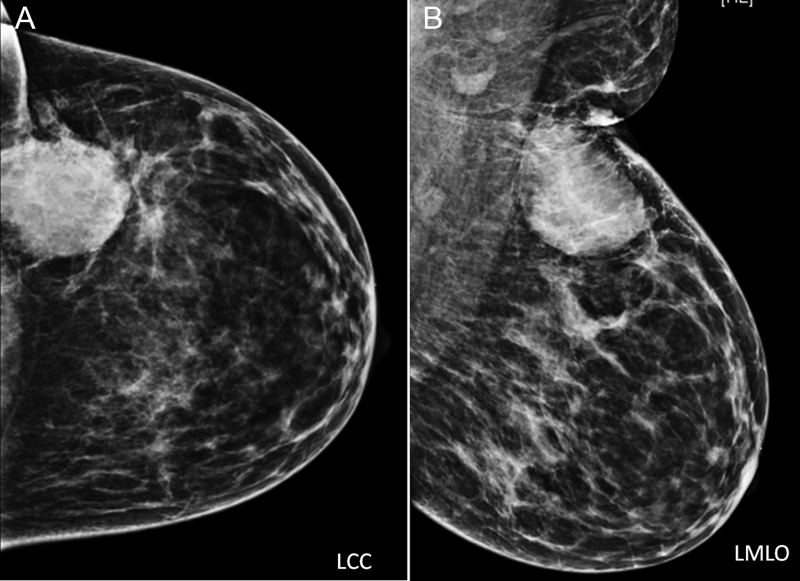
| Figure.3:Left breast LCC and LMLO views: showing a hyperdense mass in the upper outer quadrant of the left breast with partly circumscribed and partly indistinct margins. There is associated overlying skin thickening, better appreciated on MLO view. Few suspicious appearing left axillary lymph nodes are seen. No associated calcifications. Also, note heterogeneously dense breast parenchyma. Further evaluation with US is performed, to evaluate mass further and also look for any satellite lesion. LCC, left craniocaudal; LMLO, left mediolateral oblique; MLO, mediolateral oblique; US, ultrasound.
Depending on the category assigned, mass is either further addressed with core biopsy for histopathology or is kept on observation for short-term follow-ups.
Case 2
In the next case, on evaluation of mammogram, an area of asymmetry is seen.
How do we evaluate asymmetry? We can obtain an additional “spot compression view” on the mammogram, to confirm it. Many times, these areas of asymmetry resolve by spots compression view, as they exist due to overlapping breast parenchyma. Recently, with the increased availability of DBT, asymmetries are evaluated better using it. If there is the resolution of asymmetry on DBT, it confirms to be due to overlap parenchyma. If there is a persistent area of asymmetry, then the patient is evaluated further using the US, to find a US correlate. If the US examination shows abnormality, it can be biopsied using US guidance. If US is negative, it can be biopsied using stereotactic guidance in the mammography suite.
Case 3
A 50-year-old lady complains of bloody nipple discharge.
The investigation of choice is mammography. On the evaluation of mammograms, we see grouped pleomorphic microcalcifications in segmental distribution without associated mass.
This patient needs a histopathological evaluation of these calcifications. These calcifications can be biopsied in mammography suite using stereotactic guidance.
Case 4
Here, a 25-year-old lady complains of a new-onset lump in her breast.
The investigation of choice in this case is US and not a mammogram. If there is any suspicious abnormality seen in the US, then a mammogram can be performed later to look for calcifications.
As in young females the breast is very dense, and a dense mammogram has low sensitivity for the detection of abnormality. In US, mass is evaluated depending on its size, shape, margin, orientation, posterior features, vascularity, and elastography.
Case 5
Lady with BI-RADS 0 assessment on screening mammogram.
BI-RADS 0 is a category of assessment used for screening examination in asymptomatic patients. This category of assessment is not used for diagnostic evaluation in clinically symptomatic cases. If certain findings are detected on diagnostic mammogram, the patient is called back for further evaluation, either with additional mammographic views and/or with US. Once complete assessment is done, a final BI-RADS assessment category is assigned for combined mammographic and US findings. Additional investigation with MRI or CEM can be advised, but mammography assessment cannot be BI-RADS 0 in such case.
In short, for women less than 30 years of age, the first investigation of choice is US, above 40 years the investigation of choice is the mammogram. Between 30 and 40 years of age, depending on clinical presentation the first investigation will vary.
In all cases, depending on the findings of US and mammogram, further evaluation with a CEM or MRI can be performed. Histopathology of the lesion needs to be performed using appropriate modality guidance.
Brief Approach in Interpretation of Mammogram
Mammograms are routinely performed in two views, cranio-caudal view and medio-lateral oblique view). These views are not orthogonal to each other but are obtained to evaluate the maximum of breast parenchyma. Once the abnormality is detected on one view of the breast, we need to localize in the other view to predict its location in the breast ([Fig. 4A] and [B]). This is not so important for palpable abnormality but of extreme importance in non-palpable abnormality. Once we localize the abnormality on mammograms, we can evaluate it further with additional views on mammograms if needed or/and with US.
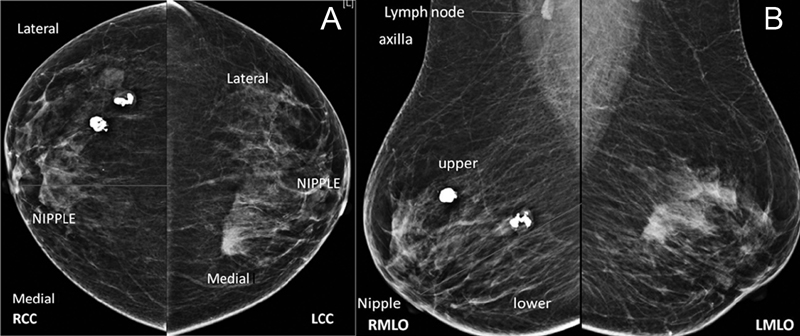
| Figure.4:(A and B): Right and left breast mammograms in CC and MLO views. On the right side, popcorn calcifications of involuting fibroadenoma are seen in the outer region on the CC view and in the upper region in the MLO view on the right side. So, they are located in the upper outer quadrant in the right breast, whereas ill-defined high-density mass on the left side is located in the upper inner quadrant. CC, craniocaudal; CEM, contrast-enhanced
Masses can be evaluated on mammogram with respect to its density, shape, and margin. Associated features like calcification, architectural distortion, skin, and trabecular thickening also need to be mentioned.
Calcifications develop within the ductal lumen within the debris of dead apoptotic cells in DCIS.[11] Calcifications that are smaller than 0.5 mm in size on mammograms are called microcalcifications. Pleomorphism in calcification means differences in size, shape, and density. So, calcifications that show this pleomorphism and are smaller than 0.5 mm are called pleomorphic microcalcifications. Along with appearance, the distribution of microcalcifications determines their significance. Linear and segmental distribution patterns of calcification have higher positive predictive values for malignancy as they are likely to be within the ductal system.
Architectural distortion seen on mammogram appears as the area of distorted Cooper's ligaments like spokes of wheel type of pattern of distortion on mammogram.
Asymmetries can be classified as asymmetry (seen only on one view), focal asymmetry (seen on both views and is small in size), global asymmetry (involving two or more quadrants), or developing asymmetry (new-onset asymmetry on mammogram). Among all asymmetries, developing asymmetry has high predictive value for malignancy.
Associated features evaluated on mammogram include skin thickening and retraction, and nipple–areolar complex abnormalities. Axillary lymph nodes if seen should also be mentioned.
All breast lesions are reported according to BI-RADS lexicons. BI-RADS lexicons are developed by ACR to communicate risk assessment and to have quality assurance. BI-RADS lexicons are developed for mammography, US, and MRI. Currently, we are using the 5th edition for reporting which was revised in 2013.[12]
Breast imaging studies are assigned one of seven assessment categories:
-
BI-RADS 0: incomplete
-
- need additional imaging evaluation (additional mammographic views or US) and/or,
-
- for mammography, obtaining previous images not available at the time of reading
-
-
BI-RADS 1: negative
-
- symmetrical and no masses, architectural distortion, or suspicious calcifications
-
-
BI-RADS 2: benign
-
- 0% probability of malignancy
-
-
BI-RADS 3: probably benign
-
- <2>
-
- short interval follow-up suggested
-
-
BI-RADS 4: suspicious for malignancy
-
- 2–94% probability of malignancy
-
- for mammography and US, these can be further divided:
-
BI-RADS 4A: low suspicion for malignancy (2–9%)
-
BI-RADS 4B: moderate suspicion for malignancy (10–49%)
-
BI-RADS 4C: high suspicion for malignancy (50–94%)
-
-
- biopsy should be considered
-
-
BI-RADS 5: highly suggestive of malignancy
-
- >95% probability of malignancy
-
- appropriate action should be taken
-
-
BI-RADS 6: known biopsy-proven malignancy
In conclusion, breast imaging has developed over the last few decades; however, it has taken a significant leap in the last decade. In this article, we have tried to briefly cover the evolution of breast imaging and some common clinical scenarios along with the approach to interpreting the mammography.
Conflict of Interest
None declared.
References
- Egan RL. Mammography. IL: Springfield “Mammography Springfield”; 1964
- Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology 1960; 75: 894-900
- Ostrum BJ, Becker W, Isard HJ. Low-dose mammography. Radiology 1973; 109 (02) 323-326
- Shapiro S. Periodic screening for breast cancer: the HIP Randomized Controlled Trial. Health Insurance Plan. J Natl Cancer Inst Monogr 1997; (22) 27-30
- Food and Drug Administration. Accessed on January 24, 2022 at: https://www.fda.gov/Radiation-Emitting-Products/Mqsa-Insights/Dbt-Accreditation-Its-Here
- Chong A, Weinstein SP, McDonald ES, Conant EF. Digital breast tomosynthesis: concepts and clinical practice. Radiology 2019; 292 (01) 1-14
- Conant EF, Barlow WE, Herschorn SD. et al; Population-based Research Optimizing Screening Through Personalized Regimen (PROSPR) Consortium. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol 2019; 5 (05) 635-642
- Ghaderi KF, Phillips J, Perry H, Lotfi P, Mehta TS. Contrast-enhanced mammography: current applications and future directions. Radiographics 2019; 39 (07) 1907-1920
- Dempsey PJ. The history of breast ultrasound. J Ultrasound Med 2004; 23 (07) 887-894
- Chang JM, Won JK, Lee KB, Park IA, Yi A, Moon WK. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am J Roentgenol 2013; 201 (02) W347-56
- Chen PH, Ghosh ET, Slanetz PJ, Eisenberg RL. Segmental breast calcifications. AJR Am J Roentgenol 2012; 199 (05) W532-42
- Breast Imaging Reporting & Data System | American College of Radiology. Published 2013. Accessed September 29, 2021 at: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
Address for correspondence
Publication History
Article published online:
15 February 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Fig. 1(A and B) With Illustration 1: bilateral mammogram in CC and MLO views. It shows two popcorn calcifications in the upper outer quadrants of the right breast suggestive of involuting fibroadenomas. A high-density mass in the upper inner quadrant of the left breast is seen.

| Fig.2:Mammogram (= low-density image on CEM) and processed contrast-enhanced images of the right breast in CC and MLO views, showing enhancement of lesion in the upper outer quadrant with suppression of background parenchymal density. Adjacent satellite lesion is better seen on contrast image (CEM) on CC view. CC, craniocaudal; CEM, contrast-enhanced mammogram; MLO; mediolateral oblique.

| Figure.3:Left breast LCC and LMLO views: showing a hyperdense mass in the upper outer quadrant of the left breast with partly circumscribed and partly indistinct margins. There is associated overlying skin thickening, better appreciated on MLO view. Few suspicious appearing left axillary lymph nodes are seen. No associated calcifications. Also, note heterogeneously dense breast parenchyma. Further evaluation with US is performed, to evaluate mass further and also look for any satellite lesion. LCC, left craniocaudal; LMLO, left mediolateral oblique; MLO, mediolateral oblique; US, ultrasound.

| Figure.4:(A and B): Right and left breast mammograms in CC and MLO views. On the right side, popcorn calcifications of involuting fibroadenoma are seen in the outer region on the CC view and in the upper region in the MLO view on the right side. So, they are located in the upper outer quadrant in the right breast, whereas ill-defined high-density mass on the left side is located in the upper inner quadrant. CC, craniocaudal; CEM, contrast-enhanced
References
- Egan RL. Mammography. IL: Springfield “Mammography Springfield”; 1964
- Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology 1960; 75: 894-900
- Ostrum BJ, Becker W, Isard HJ. Low-dose mammography. Radiology 1973; 109 (02) 323-326
- Shapiro S. Periodic screening for breast cancer: the HIP Randomized Controlled Trial. Health Insurance Plan. J Natl Cancer Inst Monogr 1997; (22) 27-30
- Food and Drug Administration. Accessed on January 24, 2022 at: https://www.fda.gov/Radiation-Emitting-Products/Mqsa-Insights/Dbt-Accreditation-Its-Here
- Chong A, Weinstein SP, McDonald ES, Conant EF. Digital breast tomosynthesis: concepts and clinical practice. Radiology 2019; 292 (01) 1-14
- Conant EF, Barlow WE, Herschorn SD. et al; Population-based Research Optimizing Screening Through Personalized Regimen (PROSPR) Consortium. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol 2019; 5 (05) 635-642
- Ghaderi KF, Phillips J, Perry H, Lotfi P, Mehta TS. Contrast-enhanced mammography: current applications and future directions. Radiographics 2019; 39 (07) 1907-1920
- Dempsey PJ. The history of breast ultrasound. J Ultrasound Med 2004; 23 (07) 887-894
- Chang JM, Won JK, Lee KB, Park IA, Yi A, Moon WK. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am J Roentgenol 2013; 201 (02) W347-56
- Chen PH, Ghosh ET, Slanetz PJ, Eisenberg RL. Segmental breast calcifications. AJR Am J Roentgenol 2012; 199 (05) W532-42
- Breast Imaging Reporting & Data System | American College of Radiology. Published 2013. Accessed September 29, 2021 at: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads


 PDF
PDF  Views
Views  Share
Share

