Broncho-alveolar Lavage Cytology Evidence of Pulmonary Metastasis by Neuroblastoma
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(06): 605-606
DOI: DOI: 10.1055/s-0045-1810428
Introduction
Neuroblastoma (NB) is the most frequent pediatric extracranial solid tumor. It exhibits a high metastatic rate, with approximately 70% of patients presenting with metastasis at diagnosis. While bone and liver are the most common sites, lung involvement occurs in 1.3 to 6.6% of cases.[1] [2] To our knowledge, this report describes the first instance of NB pulmonary metastasis diagnosed using broncho-alveolar lavage (BAL) samples.
Authors' Contributions
S.B.: concept, data acquisition, manuscript preparation, manuscript editing, and manuscript review.
V.G.V.: design, definition of intellectual content, data analysis, statistical analysis, manuscript editing, and manuscript review.
B.C.: clinical studies, experimental studies, data acquisition, and manuscript review.
G.G.: literature search, definition of intellectual content, manuscript preparation, manuscript editing, and manuscript review.
All authors have read and approved the manuscript, meet the requirements for authorship, and believe the manuscript represents honest work.
Patient's Consent
Patient's consent is not required.
Publication History
28 July 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Broncho-alveolar Lavage in Lung TransplantationM. N. Whitelaw, The Thoracic and Cardiovascular Surgeon, 1983
- Broncho-alveolar Lavage in Lung TransplantationM. N. Whitelaw, Thorac Cardiovasc Surg, 1983
- Bronchoalveolar Lavage in Other Interstitial Lung DiseasesUlrich Costabel, Seminars in Respiratory and Critical Care Medicine, 2007
- Bronchoalveolar Lavage in Other Interstitial Lung DiseasesUlrich Costabel, Semin Respir Crit Care Med, 2007
- Cytopathological examination of bronchoalveolar lavage fluid in diagnosis of pulmonary alveolar proteinosisManjari Kishore, J Lab Physicians, 2018
- Peroxiredoxin 4 as a switch regulating PTEN/AKT axis in alveolar macrophages activation<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Purine nucleoside phosphorylase dominates Influenza A virus replication and host hyperinflammation through purine salvage<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Evolving landscape of treatments targeting the microenvironment of liver metastases in non-small cell lung cancer<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Colony-stimulating factor 3 as a key mediator in the progression of idiopathic pulmonary fibrosis: a novel therapeutic target<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Introduction
Neuroblastoma (NB) is the most frequent pediatric extracranial solid tumor. It exhibits a high metastatic rate, with approximately 70% of patients presenting with metastasis at diagnosis. While bone and liver are the most common sites, lung involvement occurs in 1.3 to 6.6% of cases.[1] [2] To our knowledge, this report describes the first instance of NB pulmonary metastasis diagnosed using broncho-alveolar lavage (BAL) samples.
Case History
A 1-year-old female pediatric patient previously diagnosed with abdominal MYC-N-amplified NB presented 8 months later with dyspnea and fever. Chest scan by computed tomography revealed multiple bilateral parenchymal thickenings, suggestive of infection. A BAL was performed. The recovered fluid was processed using the cell-block (CB) method, involving formalin fixation, centrifugation, agar pre-embedding, and paraffin embedding.[3] Three-micron sections were stained with hematoxylin and eosin.
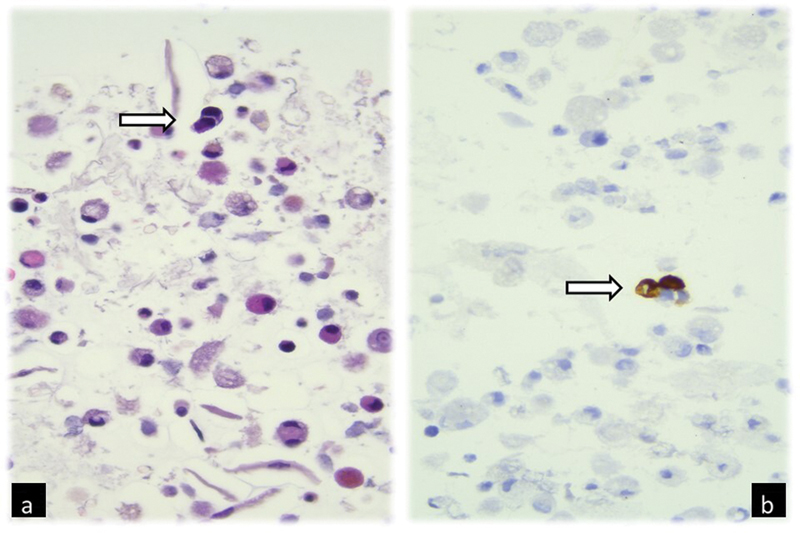
Fig 1: Broncho-alveolar lavage cell-block photomicrograph showing (a) two neoplastic cells with altered nuclear–cytoplasmic ratio and nuclear hyperchromasia (arrow) among macrophages and exfoliation cells (H-E, 400×); (b) neoplastic cells (arrow) positive for tyrosine-hydroxylase immunohistochemistry, characteristic of neuroblastoma (400×)
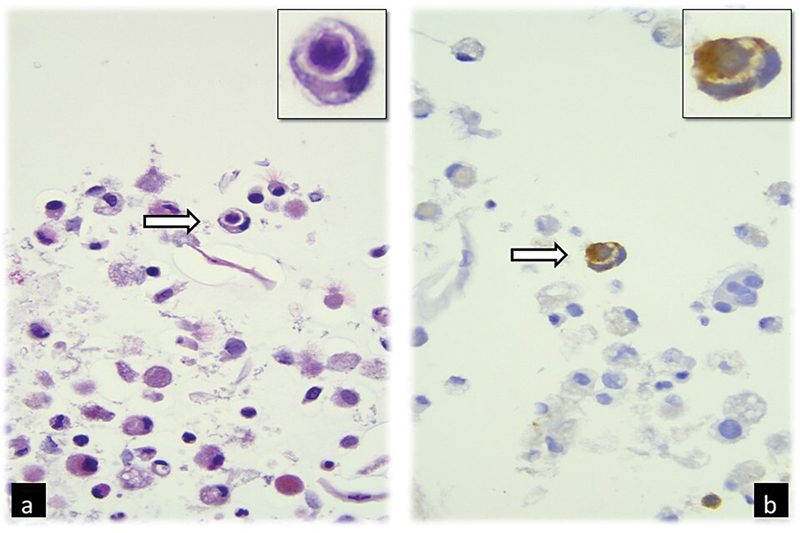
Fig 2 : Broncho-alveolar lavage cell-block photomicrograph showing (a) a cannibalistic neoplastic cell with semilunar nucleus and cytoplasm containing the engulfed cell (arrow and insert, H-E, 400×); (b) the correspondent positivity for Synaptophysin immunohistochemistry, characteristic of neuroblastoma (arrow and insert, 400×).
Discussion
The peculiarity of this case lies in diagnosing NB pulmonary metastasis via a minimally invasive BAL procedure. BAL, introduced in 1974, allows the collection of cells and secretions from the distal airways, providing valuable diagnostic information.[4]
The patient's prior NB diagnosis included MYC-N amplification, a marker of poor prognosis. Microscopically, MYC-N-amplified NBs often show enlarged and prominent nucleoli ([Fig. 3]).[5] The BAL sample maintained these microscopic features.
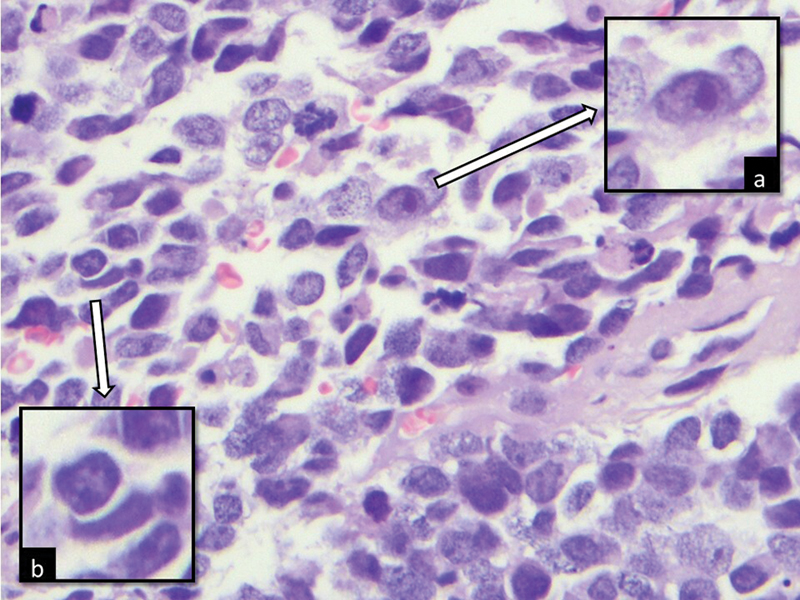
Fig 3 : Histologic photomicrograph showing a neoplastic cell with an enlarged and prominent nucleolus typical of MYC-N-amplified neuroblastoma (insert a) and a “cannibalistic” neoplastic cell (insert b) (H-E, 400 × ).
We also noted cell “cannibalism,” a survival mechanism activated under stress, where neoplastic cells engulf others.[6] While not specific to NB, its presence raised suspicion. Since only a few cells displayed these features amidst numerous macrophages, immunohistochemistry via the CB technique was essential for confirmation. This is particularly important in cases with limited cellularity, where traditional histologic analysis might be challenging. The differential diagnosis of small blue round cell tumors in cytology can be broad, necessitating the use of immunohistochemistry to accurately classify the tumor.
The main strength of this report is its demonstration that BAL combined with immunocytochemistry can enable a rapid and minimally invasive diagnosis of NB pulmonary metastasis, even in samples with limited cellularity. However, because it is based on a single case, the report's findings are limited in scope and may not be generalizable to all patients or settings. Further studies are needed to assess the sensitivity and specificity of BAL for this purpose, as well as to define its role compared with other diagnostic methods. Our approach may be restricted to specialized centers with cytopathology expertise. There are still uncertainties regarding the optimal indications for BAL and its diagnostic reliability in cases with very few tumor cells. This highlights areas for future research.
Conclusion
In conclusion, we emphasize the importance of the BAL technique, microscopic characteristics, and immunohistochemical confirmation in diagnosing NB lung metastasis. This case represents the first reported instance of NB lung metastasis diagnosed using BAL, enabling a faster diagnosis and tailored therapy while avoiding more invasive procedures.
Conflict of Interest
None declared.
Acknowledgment
The authors would like to thank technician Chiara Concetti for carrying out the cell-block technique in an optimal way.
Authors' Contributions
S.B.: concept, data acquisition, manuscript preparation, manuscript editing, and manuscript review.
V.G.V.: design, definition of intellectual content, data analysis, statistical analysis, manuscript editing, and manuscript review.
B.C.: clinical studies, experimental studies, data acquisition, and manuscript review.
G.G.: literature search, definition of intellectual content, manuscript preparation, manuscript editing, and manuscript review.
All authors have read and approved the manuscript, meet the requirements for authorship, and believe the manuscript represents honest work.
References
- WHO Classification of Tumours Editorial Board. Paediatric Tumours. 5th ed.. Lyon: IARC; 2022
- Liu S, Yin W, Lin Y. et al. Metastasis pattern and prognosis in children with neuroblastoma. World J Surg Oncol 2023; 21 (01) 130
- Krogerus L, Kholová I. Cell block in cytological diagnostics: review of preparatory techniques. Acta Cytol 2018; 62 (04) 237-243
- Patel PH, Antoine MH, Ullah S. Bronchoalveolar Lavage. Treasure Island, FL: StatPearls Publishing; 2022
- Kobayashi C, Monforte-Munoz HL, Gerbing RB. et al. Enlarged and prominent nucleoli may be indicative of MYCN amplification: a study of neuroblastoma (Schwannian stroma-poor), undifferentiated/poorly differentiated subtype with high mitosis-karyorrhexis index. Cancer 2005; 103 (01) 174-180
- Kale A. Cellular cannibalism. J Oral Maxillofac Pathol 2015; 19 (01) 7-9
Address for correspondence
28 July 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Broncho-alveolar Lavage in Lung TransplantationM. N. Whitelaw, The Thoracic and Cardiovascular Surgeon, 1983
- Broncho-alveolar Lavage in Lung TransplantationM. N. Whitelaw, Thorac Cardiovasc Surg, 1983
- Bronchoalveolar Lavage in Other Interstitial Lung DiseasesUlrich Costabel, Seminars in Respiratory and Critical Care Medicine, 2007
- Bronchoalveolar Lavage in Other Interstitial Lung DiseasesUlrich Costabel, Semin Respir Crit Care Med, 2007
- Cytopathological examination of bronchoalveolar lavage fluid in diagnosis of pulmonary alveolar proteinosisManjari Kishore, J Lab Physicians, 2018
- Ism1 deficiency in mice exacerbates bleomycin-induced pulmonary fibrosis with enhanced cellular senescence and delayed fibrosis resolution<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Peroxiredoxin 4 as a switch regulating PTEN/AKT axis in alveolar macrophages activation<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Purine nucleoside phosphorylase dominates Influenza A virus replication and host hyperinflammation through purine salvage<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Colony-stimulating factor 3 as a key mediator in the progression of idiopathic pulmonary fibrosis: a novel therapeutic target<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

Fig 1: Broncho-alveolar lavage cell-block photomicrograph showing (a) two neoplastic cells with altered nuclear–cytoplasmic ratio and nuclear hyperchromasia (arrow) among macrophages and exfoliation cells (H-E, 400×); (b) neoplastic cells (arrow) positive for tyrosine-hydroxylase immunohistochemistry, characteristic of neuroblastoma (400×)

Fig 2 : Broncho-alveolar lavage cell-block photomicrograph showing (a) a cannibalistic neoplastic cell with semilunar nucleus and cytoplasm containing the engulfed cell (arrow and insert, H-E, 400×); (b) the correspondent positivity for Synaptophysin immunohistochemistry, characteristic of neuroblastoma (arrow and insert, 400×).

Fig 3 : Histologic photomicrograph showing a neoplastic cell with an enlarged and prominent nucleolus typical of MYC-N-amplified neuroblastoma (insert a) and a “cannibalistic” neoplastic cell (insert b) (H-E, 400 × ).
References
- WHO Classification of Tumours Editorial Board. Paediatric Tumours. 5th ed.. Lyon: IARC; 2022
- Liu S, Yin W, Lin Y. et al. Metastasis pattern and prognosis in children with neuroblastoma. World J Surg Oncol 2023; 21 (01) 130
- Krogerus L, Kholová I. Cell block in cytological diagnostics: review of preparatory techniques. Acta Cytol 2018; 62 (04) 237-243
- Patel PH, Antoine MH, Ullah S. Bronchoalveolar Lavage. Treasure Island, FL: StatPearls Publishing; 2022
- Kobayashi C, Monforte-Munoz HL, Gerbing RB. et al. Enlarged and prominent nucleoli may be indicative of MYCN amplification: a study of neuroblastoma (Schwannian stroma-poor), undifferentiated/poorly differentiated subtype with high mitosis-karyorrhexis index. Cancer 2005; 103 (01) 174-180
- Kale A. Cellular cannibalism. J Oral Maxillofac Pathol 2015; 19 (01) 7-9


 PDF
PDF  Views
Views  Share
Share

