Childhood Primary Myelofibrosis Presented with Headache, Splenomegaly, and Severe Thrombocytosis: A Case Report
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 100-102
DOI: DOI: 10.4103/ijmpo.ijmpo_59_17
Abstract
Primary myelofibrosis (PMF) is a clonal disorder of a multipotent hematopoietic progenitor cell that occurs predominantly in the elderly age group. We report here an 11-year-old girl who presented with headache, fever, and splenomegaly. Full blood cell count revealed severe thrombocytosis. Laboratory and radiology examinations excluded the diagnosis of essential/reactive thrombocytosis. Bone marrow biopsy showed megakaryocytic hyperplasia, reticulin and collagen fibrosis, and erythroid and myeloid hypoplasia, findings compatible to PMF. The patient was put symptomatically on hydroxyurea and hydration due to thrombocytosis and platelet number decreased. Hematopoietic stem cell transplantation was scheduled to avoid delaying definitive therapy and secondary complications such as infections and transfusion dependency. To the best of our knowledge, this is the first reported case of PMF in childhood in Greece.
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Primary myelofibrosis (PMF) is a clonal disorder of a multipotent hematopoietic progenitor cell that occurs predominantly in the elderly age group. We report here an 11-year-old girl who presented with headache, fever, and splenomegaly. Full blood cell count revealed severe thrombocytosis. Laboratory and radiology examinations excluded the diagnosis of essential/reactive thrombocytosis. Bone marrow biopsy showed megakaryocytic hyperplasia, reticulin and collagen fibrosis, and erythroid and myeloid hypoplasia, findings compatible to PMF. The patient was put symptomatically on hydroxyurea and hydration due to thrombocytosis and platelet number decreased. Hematopoietic stem cell transplantation was scheduled to avoid delaying definitive therapy and secondary complications such as infections and transfusion dependency. To the best of our knowledge, this is the first reported case of PMF in childhood in Greece.
Keywords
Childhood - primary myelofibrosis - thrombocytosis
Introduction
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm characterized by bone marrow (BM) fibrosis, extramedullary hematopoiesis, leukoerythroblastosis, and peripheral blood cytopenias.[1] Children are rarely affected by this entity and presented with different clinical features from adults, who most commonly present with symptoms related to a cytokine-mediated state, such as fever, fatigue, weight loss, and night sweats. The clinical presentation in children is often relevant to their cytopenias and organomegaly, and the median age at diagnosis is 14 months.[2],[3] We report here a case of an 11-year-old girl with PMF presented with neurological symptoms related to her severe thrombocytosis.
Case Report
An 11-year-old female was admitted with complaints of headache for the past 2 weeks. The onset of headache was accompanied by fever of 39?C. Pain was located in the left parietal lobe and was described as pressing or tightening of mild-to-moderate intensity. The patient also complained of light and noise sensitivity. There was no history suggestive of any serious illness, and inflammatory markers were negative. Physical examination revealed the presence of splenomegaly and palpable lymph nodes in the neck region. Ophthalmological examination revealed a mild loss of visual acuity in the right eye and bilateral papilledema. Computed tomography scan and magnetic resonance imaging scan of the head were unremarkable, and the patient underwent lumbar puncture. Measurement of intracranial pressure established the diagnosis of benign intracranial hypertension (25 mmHg), and the patient was put on with acetazolamide.
Blood cell counts revealed thrombocytosis with 1.234.000/?L platelets (PLT), 8.8 ? 109/L white blood cells without blasts and red cell indices within normal range (Hct: 36.5%, Hb: 12.7 g/dL, MCV: 85.9 gL, MCH: 29.9 pg, and MCHC: 34.9 g/dL). Biochemical tests indicated high levels of lactate dehydrogenase (633U/I). Abdominal ultrasonography demonstrated no other abnormality besides splenomegaly, with a spleen of a maximum diameter of 15.3 cm and normal echogenicity. The patient underwent a BM aspiration and BM biopsy. BM biopsy demonstrated megakaryocytic hyperplasia, with megakaryocytes increased in numbers and forming clusters. They were considerable in size and had abnormal morphology with polymorphic, lobulated, bulbous, or hyperchromatic nuclei [Figure 1]a. Abnormal megakaryocytes were often found within dilated sinuses [Figure 1]b. The myeloid cell line as well as the erythroid cell line presented decrease in numbers accompanied by left shift. There were also increased BM reticulin fibers [Figure 1]c. Fine collagen fibers were highlighted by Masson stain [Figure 1]d. Immunochemistry revealed no increase in blasts or other signs indicative of leukemic progression and the diagnosis of acute leukemia was excluded. Cytogenetic and FISH analysis revealed a normal karyotype with the absence of monosomy 7, trisomy 8 and 5q- In addition, investigations for?JAK2?mutation and BCR/ABL were negative. Sequencing for the existence of genetic alterations in CALR, MPL, RUNX mutations and VPS 45 gene was not performed due to the limited amounts of DNA extracted from BM specimens. No chromosomal abnormalities were identified, except for a normal variation in chromosome 14. Vitamin D deficiency and underlying inflammation were ruled out. Liver and renal function was within normal. The diagnosis of PMF was established. The patient was put symptomatically on hydroxyurea and hydration due to thrombocytosis and PLT number decreased. Hematopoietic stem cell transplantation (HSCT) has been scheduled.
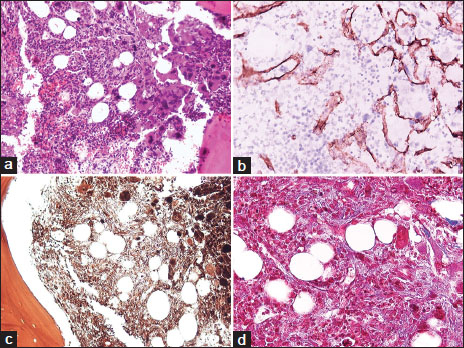
|?Figure.1Bone marrow histological findings characterized by proliferation of atypical megakaryocytes, (a) intrasinusoidal megakaryocytes and hematopoietic cells, (b) increase reticulin fibers, (c) fine collagen fibers. (d) ([a] HE, ?200; [b] CD34 immunostain, ?400; [c] Gomori stain, ?200; [d] Masson stain, ?400)
Discussion
BM fibrosis is a rare entity in children. Most frequently, fibrosis occurs secondary to other malignancies clonal myeloid or not such as acute myeloid leukemia and Hodgkin disease, chronic renal failure, infections such as tuberculosis, bone diseases such as osteopetrosis, toxins, Vitamin D deficiency, and autoimmune disorders.[3] In the present case, laboratory and radiology examinations excluded the diagnosis of essential/reactive thrombocytosis. Thus, it was termed primary.
Although somatic mutation of?JAK2?V617F, which encodes a tyrosine kinase necessary for normal hematopoiesis, is found in the BM in approximately 50% of older patients, the presence of this mutation in pediatric patients is not elucidated.[2] A 15-month-old child was the first reported case in the literature with?JAK2?mutation.[4] In the present case, the?JAK2?mutation analysis was negative. The RUNX mutation is reported in the literature to be detected in one child with PMF, it was considered premalignant change, and the patient underwent HSCT.[2] In a retrospective study, which analyzed the clinical and genetic features of Chinese pediatric patients with PMF, CALR mutation was detected in 50% of the patients.[5] Cytogenetic abnormalities are rarely detected in children with PMF, except for trisomy 21.[6] In our patient, no chromosomal abnormalities were identified, only a normal variation in chromosome 14.
The BM biopsy performed in our patient and revealed megakaryocytic hyperplasia and moderate reticulin fibrosis. The observed characteristics of megakaryocytic atypia included hyperlobation, polymorphic and mainly hyperchromatic nuclei, rather than the hypolobation, the separation of nuclear lobes, and the presence of the micromegakaryocytes, which are typically described in pediatric cases of PMF.[2],[5]
Due to the fact that PMF in childhood is an uncommon condition, there is no specific treatment approach. Thus, several cases are reported in the literature to undergo spontaneous remission, to be successfully treated symptomatically or with intravenous high dose of methylprednisolone.[2],[4],[6],[7] Nevertheless, the only curative treatment is HSCT, which leads to complete resolution of myelofibrosis with normal hematopoiesis.[3] According to the 2017 guidelines of PMF in adults, observation alone is a reasonable treatment strategy for asymptomatic low- or intermediate-1-risk disease, especially in the absence of high-risk mutations, and all the other patients should be considered for HSCT.[8]
It is concluded that children with PMF often present with severe manifestations while no molecular testing is available to establish the diagnosis of pediatric PMF. Thus, it is difficult to predict the disease progress. According to the literature, most of the children require HSCT and have good outcome overall.[3] Primary pediatric myelofibrosis should be considered in a child who presents with thrombocytosis, splenomegaly, and findings compatible to myelofibrosis on BM aspiration after exclusion of secondary causes and confirmed on BM biopsy.
Conflict of Interest
There are no conflicts of interest.
References
- Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A.?et al.?The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev 2016; 30: 453-9
- DeLario MR, Sheehan AM, Ataya R, Bertuch AA, Vega 2nd C, Webb CR.?et al?Clinical, histopathologic, and genetic features of pediatric primary myelofibrosis ? An entity different from adults. Am J Hematol 2012; 87: 461-4
- Hofmann I.?Myeloproliferative neoplasms in children. J Hematop 2015; 8: 143-57
- Maia RC, Bonamino MH, Robaina MC, Amaral N, Bonecker S, Zalcberg IR.?et al.?An unusual long-term outcome of a child with primary myelofibrosis harboring a?JAK2?mutation. Blood Cells Mol Dis 2015; 55: 347-50
- An W, Wan Y, Guo Y, Chen X, Ren Y, Zhang J.?et al.?CALR mutation screening in pediatric primary myelofibrosis. Pediatr Blood Cancer 2014;61:2256-62. Pediatr Blood Cancer 2014; 41: 2256-62
- Noor-Fadzilah Z, Leong CF, Sabariah MN, Cheong SK.?et al.?Childhood idiopathic myelofibrosis: A case report and review of literature. Malays J Pathol 2009; 31: 129-32
- Ceting?l N, Yener E, Oztop S, Nisli G, Soydan S.?Agnogenic myeloid metaplasia in childhood: A report of two cases and efficiency of intravenous high dose methylprednisolone treatment. Acta Paediatr Jpn 1994; 36: 697-700
- Tefferi A.?Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 1262-71
Address for correspondence
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure.1Bone marrow histological findings characterized by proliferation of atypical megakaryocytes, (a) intrasinusoidal megakaryocytes and hematopoietic cells, (b) increase reticulin fibers, (c) fine collagen fibers. (d) ([a] HE, ?200; [b] CD34 immunostain, ?400; [c] Gomori stain, ?200; [d] Masson stain, ?400)
References
- Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A.?et al.?The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev 2016; 30: 453-9
- DeLario MR, Sheehan AM, Ataya R, Bertuch AA, Vega 2nd C, Webb CR.?et al?Clinical, histopathologic, and genetic features of pediatric primary myelofibrosis ? An entity different from adults. Am J Hematol 2012; 87: 461-4
- Hofmann I.?Myeloproliferative neoplasms in children. J Hematop 2015; 8: 143-57
- Maia RC, Bonamino MH, Robaina MC, Amaral N, Bonecker S, Zalcberg IR.?et al.?An unusual long-term outcome of a child with primary myelofibrosis harboring a?JAK2?mutation. Blood Cells Mol Dis 2015; 55: 347-50
- An W, Wan Y, Guo Y, Chen X, Ren Y, Zhang J.?et al.?CALR mutation screening in pediatric primary myelofibrosis. Pediatr Blood Cancer 2014;61:2256-62. Pediatr Blood Cancer 2014; 41: 2256-62
- Noor-Fadzilah Z, Leong CF, Sabariah MN, Cheong SK.?et al.?Childhood idiopathic myelofibrosis: A case report and review of literature. Malays J Pathol 2009; 31: 129-32
- Ceting?l N, Yener E, Oztop S, Nisli G, Soydan S.?Agnogenic myeloid metaplasia in childhood: A report of two cases and efficiency of intravenous high dose methylprednisolone treatment. Acta Paediatr Jpn 1994; 36: 697-700
- Tefferi A.?Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 1262-71


 PDF
PDF  Views
Views  Share
Share

