Cisplatin based chemotherapy in patients with advanced differentiated thyroid carcinoma refractory to I131 treatment
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 234-237
DOI: DOI: 10.4103/0971-5851.125233
Abstract
Purpose: The purpose of the study was to evaluate the activity and toxicity of cisplatin based chemotherapy in patients with advanced differentiated thyroid cancer refractory to radioactive iodine (I131). Patients and Methods: We retrospectively reviewed the records of 20 patients with advanced thyroid cancer treated with cyclophosphamide 500 mg/m 2 on day 1, doxorubicin 50 mg/m 2 day 1 and cisplatin 50 mg/m 2 day 1 of a 21 days cycle community-acquired pneumonia (CAP) or the same regimens without cyclophosphamide adriamycin and Platinum (AP). Results: Median age of patients was 65 years (range 54-80). The majority of patients had lung metastases (60%). 4 (20%) patients achieved a partial response. Stable disease was achieved in 6 (30%) patients. Overall clinical response was 50%. Mean follow-up time was 8 months (range 4-17). Mean progression free survival was 6 months (range 3-12). Mean overall survival was 9 months (range 4-17). Patients with partial remission had a mean time to disease progression of 9 months. 4 (20%) patients had Grade 3 or 4 neutropenia. One patient had febrile neutropenia. Mild neuropathy was recorded in 5 (25%) of patients. There were no treatment related deaths. Conclusion: The combination of CAP is active in the treatment of advanced thyroid cancer with tolerable toxicity. This regimen may still be used in patients who could not be treated with targeted therapy or combined with antiangiogenic drugs in future studies.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose:
The purpose of the study was to evaluate the activity and toxicity of cisplatin based chemotherapy in patients with advanced differentiated thyroid cancer refractory to radioactive iodine (I131).
Patients and Methods:
We retrospectively reviewed the records of 20 patients with advanced thyroid cancer treated with cyclophosphamide 500 mg/m2 on day 1, doxorubicin 50 mg/m2 day 1 and cisplatin 50 mg/m2 day 1 of a 21 days cycle community-acquired pneumonia (CAP) or the same regimens without cyclophosphamide adriamycin and Platinum (AP).
Results:
Median age of patients was 65 years (range 54-80). The majority of patients had lung metastases (60%). 4 (20%) patients achieved a partial response. Stable disease was achieved in 6 (30%) patients. Overall clinical response was 50%. Mean follow-up time was 8 months (range 4-17). Mean progression free survival was 6 months (range 3-12). Mean overall survival was 9 months (range 4-17). Patients with partial remission had a mean time to disease progression of 9 months. 4 (20%) patients had Grade 3 or 4 neutropenia. One patient had febrile neutropenia. Mild neuropathy was recorded in 5 (25%) of patients. There were no treatment related deaths.
Conclusion:
The combination of CAP is active in the treatment of advanced thyroid cancer with tolerable toxicity. This regimen may still be used in patients who could not be treated with targeted therapy or combined with antiangiogenic drugs in future studies.
INTRODUCTION
Differentiated thyroid cancers (DTCs), including papillary and follicular subtypes, account for 90% of all thyroid malignancies.[1] Prognosis is generally good with long-term survival rates higher than 90%.[2] Despite their favorable prognosis patients with DTC are at risk of tumor recurrence for decades from time of diagnosis. Therapeutic management in recurrent and metastatic DTC depends on the type of initial treatment, the site and extension of the disease. Radioactive iodine (I131) administration and/or surgical excision of the lesions remain the first approach.[3] Many advanced thyroid cancers will eventually develop lack of iodine avidity, making chemotherapy the only viable option for systemic treatment. Although response rates with chemotherapy are relatively low and some metastatic patients may be referred for best supportive care, chemotherapy was the only option for symptomatic patients.[4] In the past, several single chemotherapy agents active in DTC have been recognized. Doxorubicin is an approved therapy for incurable thyroid cancer based on response rates of 20-30%.[5,6] Cisplatin and oral Tegafur Uracil UFT and other drugs have been shown to be active in treating DTC.[7,8] The relative rarity of advanced DTC refractory to I131 precluded either studies that involved a large patient population or randomized clinical trials. As a result, the comparison of the data on different multiple-agent and single-agent chemotherapeutic regimens has been extremely difficult. Later on there have been some publications on chemotherapy combinations for patients with advanced DTC.[9] The Eastern Cooperative Oncology Group (ECOG) has published a randomized study on 92 patients with advanced DTC comparing single agent doxorubicin with the combination of doxorubicin plus cisplatin.[9] Response rate was 17% in the doxorubicin group compared with 26% in the combination group.
A common element to thyroid cancers is their associated vascularity, with elevated levels of vascular endothelial growth factor (VEGF) compared with normal thyroid tissue. This is the rational after the use of antiangiogenic drugs in the treatment of advanced DTC. Both axitinib and sorafenib (tyrosine kinase inhibitors) were found to give 30% and 23% response rate retrospectively.[10,11] Most often biological targeting therapies are given alone or in combination with chemotherapy.[12] In some patients biological targeting drugs cannot be used due to their side-effects. We retrospectively summarized our results on the treatment of advanced DTC using cisplatin based combinations. The response rate and toxicity were studied. In the future, use of chemotherapy with antiangiogenic agents may improve the response rate and survival of patients with advanced DTC.
PATIENTS AND METHODS
Patients
Eligible patients were >18 years old with locally advanced or metastatic papillary or follicular thyroid carcinoma in Northern Israel. All patients were required to have progressive disease that was resistant to radioiodine therapy and to be receiving continuous thyroid suppressive therapy. All patients had inoperable disease. Patients had evaluable disease clinically or by image control and a performance status (ECOG) of three or less. Patients with a history of congestive heart failure, ventricular arrhythmia or a history of recurrent myocardial infarction were ineligible. Echocardiography was done before treatment and repeated after four courses of chemotherapy. Normal hepatic, renal and hematological functions were required. Exclusion criteria included known central nervous system metastases.
Treatment
16 patients were treated with community-acquired pneumonia (CAP): cyclophosphamide 500 mg/m2 intravenously (iv) day 1, doxorubicin 50 mg/m2 iv day 1 and cisplatin 50 mg/m2 iv day 1. Treatment was repeated every 3 weeks. Four patients were treated with AP: Doxorubicin 50 mg/m2 iv day 1 and cisplatin 50 mg/m2 iv day 1 every 3 weeks. Creatinine was evaluated every 3 weeks before starting chemotherapy. Electrocardiogram was done before each treatment. The dose of all drugs was reduced 10-20% if the patient had developed Grade 3 or 4 neutropenia in the previous cycle. Anti-emetics and analgesics were administered as appropriate.
Outcome evaluation
Physical examination was done before chemotherapy was given and repeated every 3 weeks before the administration of each course. Patients were evaluated every three cycles with computed tomography, chest X-ray and with or without bone scan. All patients were assessable for toxicity and response. Overall survival was measured from the date of starting chemotherapy until death and time to progression was measured from the date of starting chemotherapy until progression. Criteria of stopping treatment included tumor progression, serious toxicity or patient request. Following completion of treatment patients were assessed every 2-3 months until disease progression or death. Statistical analysis was performed using either Chi-square test or a Fisher exact test as appropriate.
RESULTS
In total, 20 patients were enrolled. The baseline characteristics of patients are listed in Table 1. In 18 patients, I131 was used for the treatment of the advanced or metastatic disease at a dose of 150-200 mCri every 6 or more months. Mean number of treatments with I131 was 3.2 (range 1-5). All 18 patients failed to I131 treatment. Patients having repeated treatment with I131 received chemotherapy after 6 months or more from the last date of their receiving I131. Three patients with bone metastases were treated with external radiotherapy at a 30-40 Gy dose. One patient received 60 Gy to the neck because of loco-regional disease.
Table 1
Patients characteristics (n = 20)
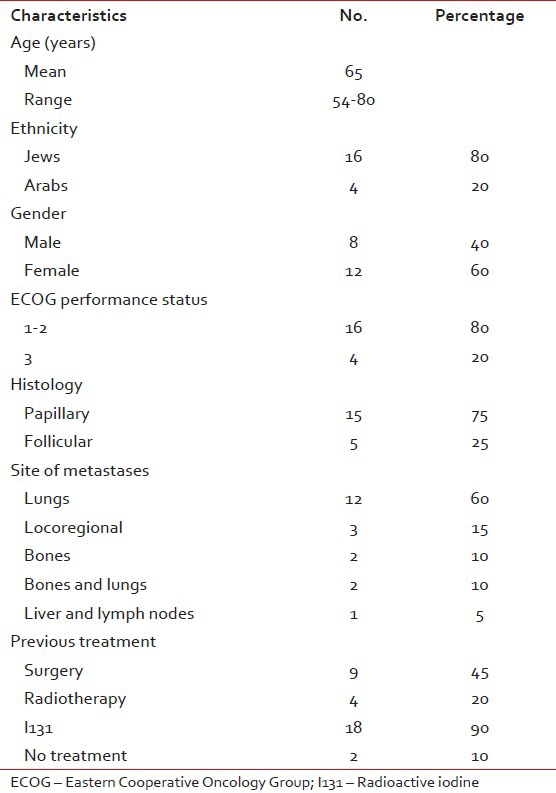
Median age of patients was 65 years [Table 1]. The majority of patients had lung metastases (60%), 15% had loco-regional recurrent disease, 10% had lung and bone, 10% had bone and 5% had liver metastases. Mean time from diagnosis of the primary tumor in the thyroid to appearance of metastases was 9.8 years (range 1-18). Mean time from metastases to starting chemotherapy was 21.5 months (range 10-36).
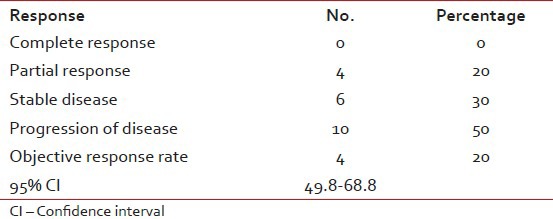
Mean number of chemotherapy cycles/patient was 4 (range 2-10). Relative dose intensity was 76%. 4 (20%) patients achieved a partial response; three of them with lung metastases and one with locoregional disease. All four patients with partial response were treated with CAP. Stable disease was achieved in 6 (30%) patients. Overall clinical response was 50% (95% confidence interval 49.8-68.9%). Mean follow-up time was 8 months (range 4-17). Mean progression free survival was 6 months (range 3-12). Mean overall survival of all patients was 9 months (range 4-17). All patients died of their disease. Patients with partial remission (20%) had a mean time to disease progression of 9 months (range 6-12) [Table 2].
Table 2
Response to treatment
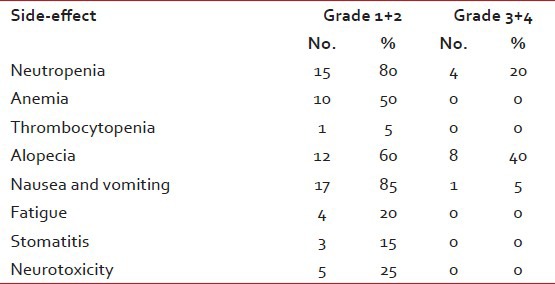
Side-effects were within the acceptable limits. The major toxicity encountered with this regimen was hematological. Table 3 contains the list of side-effects. 4 (20%) patients had Grade 3 or 4 neutropenia. One patient had febrile neutropenia treated successfully after admission to the hospital. There were no treatment related deaths. None of the patients had suffered of renal failure. Most patients had a mild nausea or vomiting. Mild reversible neuropathy was recorded in 5 (25%) of patients.
Table 3
Treatment related side-effects
DISCUSSION
Thyroid cancer is the 17th most common cancer world-wide; in 2002, the estimated incidence was more than 141,000 cases, more than three quarters of which occurred in women.[13] For differentiated tumors, standard treatment is primary surgery, thyroid stimulating hormone suppression and ablation of thyroid remnant with I131. The overall survival rate is approximately 90% at 20 years.[14,15] Although the prognosis for DTC is quite favorable I131 refractory, recurrent or metastatic disease is prognostically more worrisome and most patient survive less than 3 years with clinical symptoms.[16]
Until now, there has been no effective therapy for metastatic thyroid cancer that is not amenable to surgery and that does not concentrate iodine. Several chemotherapy agents were recognized to be active in this disease; doxorubicin, bleomycin, cisplatin and other agents.[4,5,9,17] Thereafter, there have been publications on multiple-agent chemotherapy.[9] ECOG study demonstrated higher response rate with doxorubicin and cisplatin combination compared with doxorubicin.[9] In the recent study, 20% of patients treated with doxorubicin plus cisplatin with or without cyclophosphamide had a partial response and additional 30% had stable disease. Mean progression free survival and overall survival were 6 and 9 months respectively. These results are similar to some publications in the literature.[5,9,11] Side-effects were tolerable. In the absence of a more effective treatment, this regimen can be used for patients with advanced thyroid carcinoma.
In recent years, it has been shown that the inhibition of VEGF receptor (VEGFR) signaling inhibits the growth of thyroid tumors.[18,19] Sorafenib, an orally active multikinase inhibitor with multiple targets including VEGFR, was proven to be effective in the treatment of advanced I131 refractory tumors. Partial response was reported in 23% of 30 patients treated with sorafenib, lasting for 18-89 weeks.[11] Axitinib; an antiangiogenic small molecule was also found to be effective in advanced DTC. Objective response was 30% in advanced DTC.[10] In the 2013, National Comprehensive Cancer Network This is correct expansionguidelines it is suggested to consider small molecule kinase inhibitors or systemic therapy (if trial not available) for none-radioiodine responsive tumors.[20]
International experience has shown that biological targeting drugs are more active when given with chemotherapy. Trastuzumab is used in metastatic breast cancer tumors which overexpress human epidermal growth factor receptor 2. When given alone, its’ objective response rate was 15% compared to 60% when it was used in addition to chemotherapy.[21] The same result was observed when bevacizumab was given alone or in combination with chemotherapy in advanced colorectal cancer.[12] Therefore, we believe that biological targeting drugs found to be effective in DTC may lead to higher response rates if they are given with chemotherapy.
CONCLUSION
CAP combination is moderately effective in the treatment of I131 refractory DTC, with acceptable toxicity. This regimen can still be used in patients who failed or cannot use biological targeting drugs. New studies using antiangiogenic drugs in combination with CAP would be worthwhile.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

