Clinical evaluation of total and lipid bound sialic acid levels in oral precancer and oral cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(01): 36-41
DOI: DOI: 10.4103/0971-5851.96967
Abstract
Aims: Special attention has been given to define the biochemical changes in cell-surface glycoproteins and glycolipids that take place during malignant transformation. This study was thus designed to explore the clinical utility of total and lipid bound sialic acid (LSA) in patients with oral precancer and oral cancer. Materials and Methods: Blood samples were obtained from 95 subjects divided into three groups, namely healthy individuals, oral cancer, and precancer. Serum total and LSA levels were determined using periodate-thiobarbituric acid method and Katopodis et al. method, respectively. Finally, spectrophotometricreadings were obtained. Results: Mean values of serum sialic acid (total and lipid bound) in oral cancer were significantly higher than control and the precancer group (P<0 class="b" xss=removed>Conclusion: Serum sialic acid levels can differentiate between patients with oral precancer and oral cancer. It could be used as an adjunct to diagnosis, monitor response to therapy, and assess the staging of cancer.
Keywords
Katopodis - oral cancer - oral precancer - periodate-thiobarbituric acid - sialic acid - spectrophotometerPublication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
Special attention has been given to define the biochemical changes in cell-surface glycoproteins and glycolipids that take place during malignant transformation. This study was thus designed to explore the clinical utility of total and lipid bound sialic acid (LSA) in patients with oral precancer and oral cancer.
Materials and Methods:
Blood samples were obtained from 95 subjects divided into three groups, namely healthy individuals, oral cancer, and precancer. Serum total and LSA levels were determined using periodate-thiobarbituric acid method and Katopodis et al. method, respectively. Finally, spectrophotometricreadings were obtained.
Results:
Mean values of serum sialic acid (total and lipid bound) in oral cancer were significantly higher than control and the precancer group (P<0>
Conclusion:
Serum sialic acid levels can differentiate between patients with oral precancer and oral cancer. It could be used as an adjunct to diagnosis, monitor response to therapy, and assess the staging of cancer.
INTRODUCTION
Squamous cell carcinoma is the most common cancer in the oral cavity. There are 222,000 new cases (5% of all cancers) of oral cancer diagnosed in men each year globally and 90,000 new cases (2% of all cancers) diagnosed in women. The 5-year survival rate is estimated to be about 50%.[1] In India, oral cancer ranks number one among all cancers in male patients and number three among cancers in female patients.[2] Oral cancer is preceded in many cases by oral precancer such as oral leukoplakia and oral submucous fibrosis. Early diagnosis of oral cancer as well detection of dysplastic changes in oral precancer can significantly decrease the mortality rate. The present clinical approach for cancer diagnosis and management involves invasive and painful procedures. Therefore, a simple and noninvasive tumor marker is required for early diagnosis as well as for monitoring progress during the treatment. Aberrant glycosylation in malignant or transformed cells results in increased synthesis of carbohydrates following that there is increased levels of sialic acid on their surfaces.[3,4] These glycoconjugates are released into the circulation through increased turnover, secretion, or shedding from malignant cells. Increased quantities of glycoconjugates such as total sialic acid (TSA) and lipid bound sialic acid (LSA) have been detected in the serum of head and neck cancer patients.[5–10]
The study was conducted to determine the serum levels of total and LSA in oral precancer and oral cancer, to ascertain whether serum levels of total and LSA can be used as a help in differentiating between precancerous and cancerous disease, to evaluate whether serum levels of TSA and LSA vary as per the clinical and histological grade of oral cancer.
MATERIALS AND METHODS
This study included three groups: (1) 35 age matched healthy controls with no major illness in the recent past, (2) 30 patients with oral precancer (age range: 20–80 years, average age: 39.83 years). Out of 30 patients, 15 had oral leukoplakia and 15 had oral submucous fibrosis. (3) 30 oral cancer patients (age range: 30–74 years, average age: 49.83 years). Diagnosis of oral precancer and oral cancer was based on clinical, radiological, and histopathological reports. Approval by local ethical committee was obtained before commencing the study. The determination of clinical stage of disease was as per American Joint Committee of Cancer.[11] Blood samples were collected by vein puncture, was allowed to clot, settle at room temperature and centrifuged. Serum was stored at −20°C until analyzed.
Serum TSA levels were determined using the periodate-thiobarbituric acid method.[12] Serum samples (100 μl) were hydrolyzed in 2 ml of 0.05 mol/l of H2SO4 at 80°C for 1 h. After hydrolysis proteins were precipitated by 1.0 ml of 10% trichloroacetic acid. The supernatant was incubated with 0.025 N periodic acid at 37°C for 30 min. Reaction was terminated by addition of 2% sodium arsenite. Then 6% of thiobarbituric acid was added and the mixture was kept in boiling water bath for 7.5 min. 1.5 ml of dimethyl sulphoxide was added to increase the stability of the chromophore. For each determination, spectrophotometric readings against blank were made at 550 and 532 nm to overcome any interference from 2-deoxy-D-ribose. Interassay and intraassay coefficients of variations for TSA estimations were 4.5% and 5.3%, respectively. Serum LSA levels were measured by Katopodis et al. method.[13] Serum measuring 50 μl was extracted with chloroform–methanol (2:1v/v) maintained at 4°C. The lipid extract was separated with 0.5 ml of distilled water. The aqueous was precipitated with phosphotungstic acid. The precipitate was resuspended in 1 ml of distilled water and LSA in suspension was determined with resorcinol reagent. Interassay and intraassay coefficients of variations for LSA estimations were 4.8% and 6.5%, respectively. The N-acetyl neuraminic acid as standard was obtained from Sisco Research Laboratories Ltd. for standardization of the procedure [Figures [Figures11 and and22].
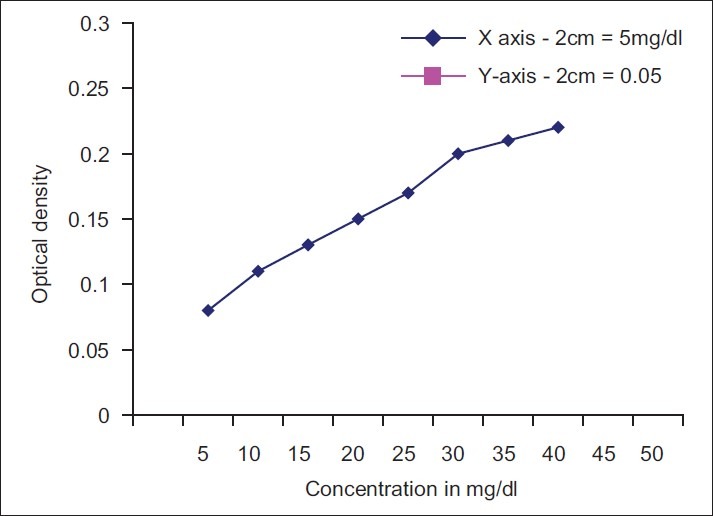
| Fig. 1 Standardization of total sialic acid
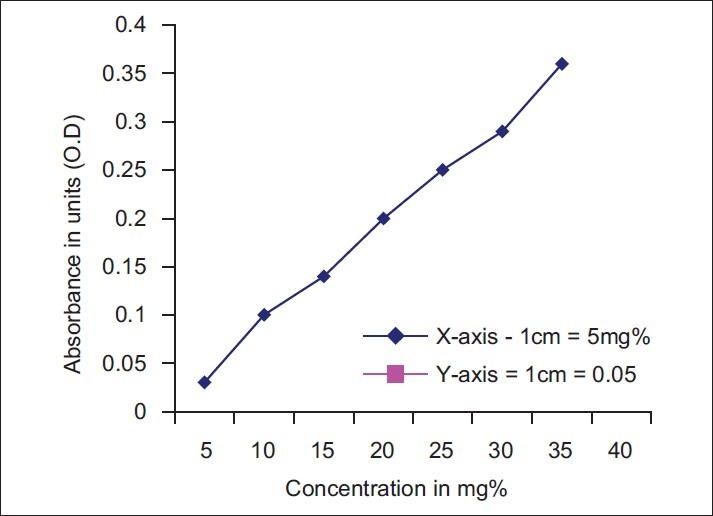
| Fig. 2 Standardization of lipid bound sialic acid
To calculate the concentration of TSA and LSA the following formula was used.

Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) 10.0 software. Student t-test, Analysis of variance (ANOVA) test were carried out for comparison between the three groups. The values of the biomarkers were expressed as the mean ± the standard deviation (SD). Multivariate analysis was performed to determine the alterations in TSA and LSA levels with the clinical stage and histopathological grade of oral cancer.
RESULTS
Table 1 shows detailsof habits of oral precancer and cancer patients. Tobacco chewing was the most common habit. Table 2 shows comparison of the levels of serum TSA and LSA between controls, patients with oral precancer, and oral cancer. The levels of TSA and LSA showed significant elevations in oral precancer patients compared with controls (P<0 xss=removed>P<0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3385277/table/T3/" target="table" class="fig-table-link figpopup" rid-figpopup="T3" rid-ob="ob-T3" co-legend-rid="" xss=removed>Table 3 shows mean±SD values for serum TSA and LSA in histopathological grade I, II, and III oral cancer patients. Difference in values of serum TSA was statistically significant between grade-I and grade-II oral cancer. However, the difference between grade-II and grade-III cases was not significant. Also, alterations in serum LSA did not show any correlation with grade of oral cancer. Multivariate analysis revealed that neither TSA nor LSA values showed any significant association with the histopathologic grade of the disease.
Table 1
Details of habits of oral precancer and cancer patients
Table 2
Comparison of total sialic acid and lipid bound sialic acid levels between healthy controls, oral precancer, and oral cancer patients
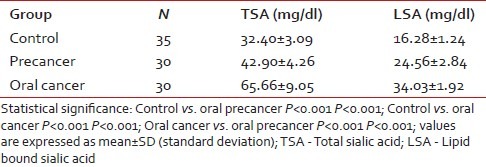
Table 3
Comparison of total sialic acid and lipid bound sialic acid levels with histopathological grade of oral cancer patients

Table 4
Comparison of total sialic acid and lipid bound sialic acid levels with clinical stages of oral cancer patients
DISCUSSION
Tumor markers are substances specific for certain tumor or cancer cells and thus could be important for diagnostic and prognostic purpose in oral cancer patients. The plasma membrane is one of the sites where genetic changes are expressed and is important for control of normal cell behavior and proliferation.[14] Nicolson in 1976 described various types of plasma membrane alterations associated with cellular transformation.[15] The plasma membrane is composed of phospholipids, glycoproteins, and glycolipids. The carbohydrate portions of these glycoproteins and glycolipids project from the outer surface of the membrane and form the cell coat. The cell coat is made up predominantly of sialic acid containing glycoproteins.[16] Sialic acid, a family of acylated derivatives of neuraminic acid, occurs as a terminal component at the nonreducing end of carbohydrate chains of glycoproteins and glycolipids.[17] Sialic acid plays an important role in cell–cell recognition, invasiveness, adhesiveness, and immunogenecity.[8] The major structural component of cell surface and glycoproteins undergoes alteration on neoplastic transformation of cell. One such alteration is an elevation in the level of sialic acid on the cell surface. These glycoconjugates are released into the circulation through increased turnover, secretion, and/or shedding from malignant cells leading to elevation of sialic acid levels in blood.
Measurement of serum sialic acid in cancer patients can serve as a diagnostic and prognostic marker in malignant diseases. Earlier studies have compared significantly elevated levels of serum sialic acid TSA, LSA in cancer patients with healthy controls. Kakari et al.[18] compared TSA and LSA levels with carcinoembryonic antigen, ferritin, and neuron specific enolase. They suggested that of the five biomarkers, TSA and LSA were the most sensitive markers for lung cancer. It is also found that different forms of sialic acid (TSA, LSA, free sialic acid, and TSA/total proteins) are more suitable for monitoring disease extent and anticancer therapy.[17]
Usually oral cancer is preceded by oral precancerous conditions such as oral leukoplakia and oral submucous fibrosis. Many follow-up studies have shown that between 0.13 and 36.4% of oral leukoplakia will develop into oral cancer after a follow-up period of 1 to 11 years.[19] Therefore, for the control of oral cancer, it is very important to detect and manage oral leukoplakia early in their development and prevent their malignant transformation. There are only few studies that have compared the alterations between oral precancer and oral cancer. In a study conducted by Baxi et al.[8] only the levels of LSA and protein bound hexoses (PBH) were elevated in oral precancer, and significantly increased levels of TSA, LSA, mucoid proteins, and PBH occurred in oral cancer, whereas in Rajpura et al.[9] both TSA and LSA showed significant elevation in patients with oral precancer and oral cancer than in healthy controls. In a study conducted by Joshi et al.[10] increase in serum TSA levels was observed in oral precancer and oral cancer compared with the control group. Harvey et al. in their study performed serial determination of sialic acid in 34 patients with widespread malignancy. They showed that all six patients who underwent surgical treatment had a drop in their serum sialic acid values to normal levels following a transient rise in the immediate postoperative period. Twenty-eight patients received chemotherapy, out of which four patients demonstrated tumor regression and in seven patients the disease was stable. The remaining 17 patients showed tumor progression. All four patients with responsive disease demonstrated a fall in serum sialic acid levels, patients with clinically stable disease showed a less marked decrease, whereas most patients with widespread progressive disease showed increase in sialic acid levels and this increase preceded clinical relapse in majority of patients. This shows that rising sialic acid values may predate clinical evidence of tumor resistance to previously effective chemotherapy, indicating a need for a change in treatment. Thus, sialic acid may also be useful as a monitor of response to chemotherapy of many cancers for which there is no available tumor marker.[7] Charushila Y et al. in their study showed that for TSA, the positive predictive value was 87% and negative predictive value of test was 89%. For LSA the predictive value of a positive test was 97%, the predictive value of negative test was 84%. They also stated that regarding the cost of the measurements, the TSA was the cheapest among the three tumor markers [β2 –microglobulin, TSA and LSA].[20]
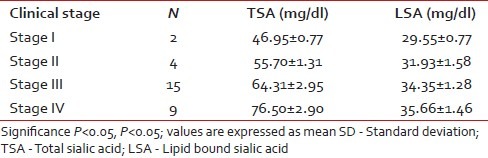
In this study, elevation in the levels of both serum TSA and LSA in oral precancer patients was statistically significant compared with the control group. Thus, elevation in TSA and LSA can give an early indication of a premalignant change. If these patients are routinely monitored, frank malignancy can be detected at an early and treatable stage. The increase in TSA and LSA levels were also statistically significant in untreated oral cancer patients compared with control and the precancer group, indicating that serum glycoconjugates can be used to clearly differentiate between oral cancer and precancer [Figure 3]. This was consistent with the findings by Baxi et al.[8] Rajpura et al.[9] and Joshi et al.[10] This shows that malignant cells have altered surface carbohydrate composition leading to aberrant cell–cell recognition resulting in breakdown of intercellular control mechanism that is in accordance with earlier reports.
| Fig. 3 Comparison of total and lipid bound sialic acid levels between control, precancer, and oral cancer
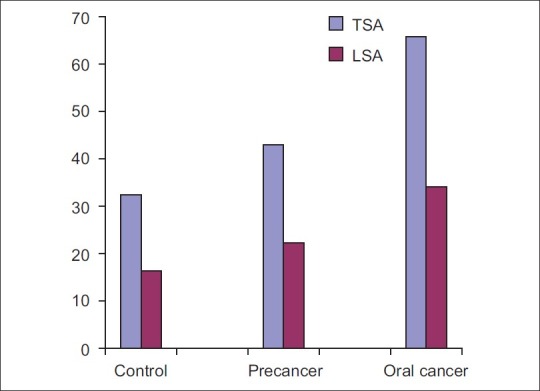
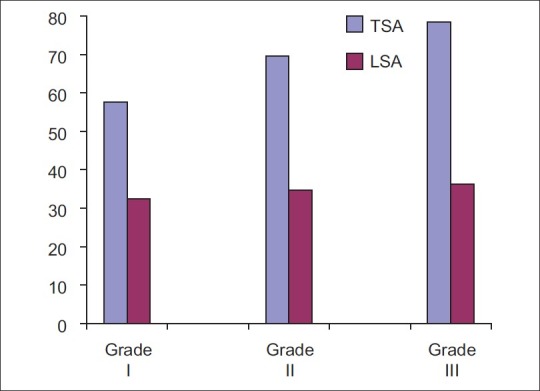
The tumor node metastasis staging and histological grading of tumor has been used for many decades in an attempt to predict the clinical behavior of oral cancer. Difference in values of serum TSA was statistically significant between grade-I and grade-II oral cancer. However, the difference between grade-II and grade-III cases was not significant [Figure 4]. The possible reason could be subjective variation between histopathologic grading or that the same tumor may show different degree of differentiation in varying areas.[21] Multivariate analysis revealed that neither TSA nor LSA showed any significant association with the histopathologic grade of the disease. Results obtained are similar to previous studyconducted by Rajpura et al.[9] and Joshi et al.[10] Serum TSA and LSA levels in oral cancer patients progressively increase from stage-I to stage-IV, the highest level found in most advanced stage of malignancy indicating that the levels are directly proportional to tumor burden and correlated well with the stage of primary lesion and presence of distant metastasis [Figure 5]. As a result, even the extent of malignant disease can be ascertained. Evaluating these markers can also help in detecting metastatic cancer in stage-IV oral cancer patients. This also indicates that higher levels of TSA and LSA may be associated with poor prognosis. The results correlated well with the studies conducted by Baxi et al.[8] Rajpura et al.[9] and Joshi et al.[10]
| Fig. 4 Comparison of total and lipid bound sialic acid levels between grade-I, grade-II, and grade-III carcinoma
| Fig. 5 Comparison of total and lipid bound sialic acid levels between clinical stages of squamous cell carcinoma
Elevated levels of serum sialic acid have been found in inflammatory diseases, rheumatoid arthritis, Crohn's disease and psoriasis.[6] Certain drugs also alter the serum sialic acid levels. Oral antidiabetic drugs, for example, metformin and rosiglitazone raise the serum sialic acid levels, while fenofibrate (reduce cholesterol levels in patients at risk of cardiovascular disease), anti-inflammatory agents such as mefanamic acid and chemotherapeutic agents lower the serum sialic acid content.[7,22,23] None of the patients in our study were either on any drugs or were suffering from any of the above-mentioned diseases that could alter the serum sialic acid level.
CONCLUSION
In conclusion, the combined evaluations of biomarkers such as TSA and LSA provide useful biochemical indices for clinical assessment of the spread and invasiveness of malignant disease of oral cavity and can differentiate between patients with oral precancer and oral cancer. Thus, serum TSA and LSA could be used as an adjunct to diagnosis, monitor response to therapy and assess the staging of cancer. The limitation of the study was the relatively small sample size. There is still scope for further study where more number of patients with larger variety of malignancies could be taken into account.
ACKNOWLEDGMENT
I am thankful to Nilanjana Guha Niyogi, Lecturer, Biochemistry department, Sir J. J. Group of Hospitals, Mumbai for estimation of serum TSA and LSA levels in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Standardization of total sialic acid


 PDF
PDF  Views
Views  Share
Share

