Clinical Profile of Pediatric Oncology Patients Treated by External Beam Radiotherapy: An Institutional Experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 28-32
DOI: DOI: 10.4103/0971-5851.203497
Abstract
Introduction: Pediatric tumors are a heterogeneous group of malignant conditions requiring multimodal treatment, and management of such cases is at time challenging. We present the clinical profile of pediatric cancer patients who received radiation, either alone or as adjuvant to surgery and chemotherapy; in prophylactic, radical or palliative clinical setting. Aim: This study was envisaged to review our experience of pediatric oncology cases, their clinical and morphological profile, dosage schedule of radiotherapy, and the therapy induced complications. Settings and Design: This was a retrospective, observational study carried out in an apex tertiary care cancer institute of government set-up in a developing country. Materials and Methods: The treatment charts and clinical summary of patients who had received radiation over the last 5 years period were retrieved and perused. Various clinical and pathological parameters were studied and inferences drawn. Results: A total of 50 patients got radiation over 5 year study-period, including 37 male and 13 female patients. The commonest age group of presentation was 8-12 years followed by 13-16 years. The mean age of presentation was 9.3 years. The most common diagnosis was hematological malignancies followed by CNS tumors with 21 and 13 patients respectively. Overall the most common indication of RT was in adjuvant setting after surgery as the definitive management, where 24 patients were irradiated; and the next common indication was prophylactic cranial irradiation in 14 patients of childhood leukemias. 10 patients tolerated treatment with Grade 1 site-specific or systemic toxicities while 7 patients developed Grade 2 and more systemic toxicities. 9 patients received craniospinal irradiation, common indications being medulloblastoma and Atypical teratoma rhabdoid tumor (ATRT). 3 patients received concurrent chemotherapy with weekly Inj Vincristine. 17 patients required sedation or short general anaesthesia for radiation planning and execution. Conclusion: External beam Radiotherapy constitutes an important component of management of pediatric cancers. One should be judicious in Radiotherapy planning, execution and monitoring acute and delayed toxicities.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Pediatric tumors are a heterogeneous group of malignant conditions requiring multimodal treatment, and management of such cases is at time challenging. We present the clinical profile of pediatric cancer patients who received radiation, either alone or as adjuvant to surgery and chemotherapy; in prophylactic, radical or palliative clinical setting.
Aim:
This study was envisaged to review our experience of pediatric oncology cases, their clinical and morphological profile, dosage schedule of radiotherapy, and the therapy induced complications.
Settings and Design:
This was a retrospective, observational study carried out in an apex tertiary care cancer institute of government set-up in a developing country.
Materials and Methods:
The treatment charts and clinical summary of patients who had received radiation over the last 5 years period were retrieved and perused. Various clinical and pathological parameters were studied and inferences drawn.
Results:
A total of 50 patients got radiation over 5 year study-period, including 37 male and 13 female patients. The commonest age group of presentation was 8-12 years followed by 13-16 years. The mean age of presentation was 9.3 years. The most common diagnosis was hematological malignancies followed by CNS tumors with 21 and 13 patients respectively. Overall the most common indication of RT was in adjuvant setting after surgery as the definitive management, where 24 patients were irradiated; and the next common indication was prophylactic cranial irradiation in 14 patients of childhood leukemias. 10 patients tolerated treatment with Grade 1 site-specific or systemic toxicities while 7 patients developed Grade 2 and more systemic toxicities. 9 patients received craniospinal irradiation, common indications being medulloblastoma and Atypical teratoma rhabdoid tumor (ATRT). 3 patients received concurrent chemotherapy with weekly Inj Vincristine. 17 patients required sedation or short general anaesthesia for radiation planning and execution.
Conclusion:
External beam Radiotherapy constitutes an important component of management of pediatric cancers. One should be judicious in Radiotherapy planning, execution and monitoring acute and delayed toxicities.
Introduction
Childhood cancer contributes to <5 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398103/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_603278051" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1,2] In the developed countries, 80% of the children with cancer are cured, but the scenario in India continues to be dismal where cure rates are estimated to be between 10% and 30%.[3,4] Pediatric oncology is an upcoming specialty in India and lots of gaps need to be filled in this arena. Childhood cancers need to be managed aggressively by multimodal therapy and need close collaboration between various clinicians involved, especially pediatric surgeons, medical oncologists and radiation oncologists.
Pediatric radiation therapy can be especially challenging in view of the risk of delayed neurocognitive and endocrine toxicities and the development of secondary cancer among the survivors. Reproductive health and fertility are of great importance to the increasing number of survivors of childhood cancer but in general, there is a deficiency in the information provided and counseling of patients and families at the time of diagnosis with regards to the risk of infertility.[5,6] Hence, such cases should be discussed jointly in a multidisciplinary tumor board clinic before starting the treatment. These patients may require frequent hospitalizations and active supportive care to manage acute therapy-induced toxicities. We present the clinical profile of pediatric cancer patients who received radiation, either alone or as adjuvant to surgery and chemotherapy; in prophylactic, radical, or palliative clinical setting.
Materials and Methods
This was a retrospective observational study conducted in Radiotherapy (RT) Department of a tertiary care multi-specialty government hospital of academic interests with a dedicated oncology center. All these patients had an underlying diagnosis of histopathologically proven malignancy and were started on RT after joint discussion and exhaustive simulation and planning exercises. The treatment charts and clinical summary of patients who had received radiation over the past 5 years were retrieved and perused. Various clinical and pathological parameters were studied and inferences drawn. A note was of age, site, histopathology, and intent of treatment. Pertinent observations were tabulated and data were analyzed. The site-specific and systemic toxicities induced by radiation were noted.
Results
A total of fifty patients got radiation over 5 years study, constituting about 1.5% of the total patients who received RT during that period. The study group comprised 37 male and 13 female patients. The most common age group of the presentation was 8–12 years followed by 13–16 years. The age-wise distribution of patients is given in Table 1. The mean age of presentation was 9.3 years. The most common diagnosis was hematological malignancies followed by central nervous system tumors with 21 and 13 patients, respectively. Among the former, the most common pathology noted was acute lymphoblastic leukemia (ALL), where 14 patients received prophylactic cranial irradiation (PCI). The site-wise presentation is tabulated in Table 2.
Table 1
Age-wise distribution

|
Table 2
Site-wise distribution
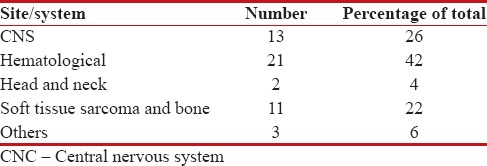
|
Overall the most common indication of RT was in the adjuvant setting after surgery as the definitive management, where 24 patients were irradiated; and the next common indication was PCI in 14 patients of childhood leukemias. The intent of treatment is given in Table 3. A total of 44 patients were treated on a linear accelerator compatible with three-dimensional conformal RT, whereas remaining were treated on Telecobalt Unit. Three patients received RT on two or more occasions, initially with radical intent followed by palliative RT.
Table 3
Intent of treatment

|
Ten patients tolerated treatment with Grade 1 site-specific or systemic toxicities, whereas 7 patients developed Grade 2 and more systemic toxicities. Three patients developed afebrile neutropenia, four patients developed febrile neutropenia and one developed pancytopenia warranting interruption of treatment. Nine patients received craniospinal irradiation, common indications being medulloblastoma and atypical teratoma rhabdoid tumor, followed by primitive neuroectodermal tumor and ependymoma. Seven patients received upfront palliative RT for advanced disseminated disease, and two patients who were initially treated with definitive RT were later offered palliative RT when they relapsed with metastatic disease. Three patients received concurrent chemotherapy with weekly injection vincristine. Seventeen patients required sedation or short general anesthesia for radiation planning and execution, majority of these were <6>
Discussion
In India, the incidence of pediatric cancers is on the increase. Satyanarayana et al., in a recent review of 25 population-based cancer registries of India, found that the age-adjusted cancer incidence rates ranged from 18.6 per million to 159.6 per million for boys and 11.3–112.4 for girls. The highest incidence was observed for males (159.6) in Southern region of the country and the lowest in Northeast in both boys (18.6) and girls (11.3).[7] In a similar study by Arora et al.[8] to ascertain the burden of childhood cancer in India, it was found that 1.6%–4.8% of all cancer in India is seen in children below 15 years of age and the overall incidence of 38–124 per million children, per year, is lower than that in the developed world. Leukemia is the most common childhood cancer in India with relative proportion varying between 25% and 40%. Worldwide, cancer in childhood is more common among males than females and the male to female ratio in the most resource-rich countries is around 1.2:1. The authors observed that some cancers such as retinoblastoma, Wilms' tumor, osteosarcoma, and germ cell tumor actually show a slight female preponderance.
Multiple inter-related factors are responsible for the poorer outlook of childhood cancer in India. Limited financial resources, lack of awareness of the meaning of symptoms, and difficulty in accessing healthcare contribute to the advanced stage of presentation. Such a delay in presentation, along with unfavorable biology leads to a need for more intense treatment, resulting in higher treatment-related morbidity and mortality.[1,8] Treatment refusal or abandonment, besides treatment-related death, is a frequent unwanted outcome. Reasons for treatment abandonment in India include financial problems, transportation difficulties, beliefs about cancer curability, fear and experience of severe side effects, child refusal (in adolescents), girl child, and dissatisfaction with the treating hospital.[9,10]
A higher 5 years survival is seen in those treated at specialist cancer centers and among those who complete their treatment, reinforcing the importance of centralization of treatment and compliance. There is paucity of certified Pediatric Oncology centers in India, and the government needs to take prompt significant steps to improve existing infrastructure and workforce status. Although child health continues to be the priority health issue, childhood cancer is not yet a major area of focus. Lots of resources are utilized in reducing childhood mortality by the promotion of breastfeeding, rational antibiotic therapy for acute respiratory infections, oral rehydration for diarrhea, an extensive immunization program, and appropriate prevention and treatment of malaria.[8]
One essential strategy to improve the oncology outcome in India should be the development of regional networks of pediatric oncology professionals, structured teaching programs of pediatric oncology among postgraduate residents, redistribution of resources for optimal utilization, promotion multicenter clinical research to address local issues and develop programs to improve outcomes at the regional level.[11] Pediatric oncology in India needs a concerted, collaborative, and multidimensional effort to reach international standards. The formation of co-operative groups and local protocols is the need of the hour. Close collaboration between various regional groups should be recommended to promote multispecialty care, to improve standards of care and to bring out consensus guidelines for management of various tumors.[12,13] It will need joint collaborative steps by physicians, researchers, epidemiologists, administrators, support groups, and all individuals dedicated to the effective treatment of childhood cancer in India.
There has been a substantial improvement in the prognosis of children with cancer with the use of aggressive treatment protocols that include a combination of chemotherapy, radiation, and surgery, but life-threatening complications can occur with these intensive treatment regimens, most prominent of which are infections that are as a result of treatment-associated immune suppression. A high incidence of sepsis has been demonstrated in pediatric patients receiving chemotherapy, approximately 12.8% in children aged 1–9 years and 17.4% in children aged 10–19 years, making febrile neutropenia a serious and worrying complication in pediatric cancer treatment.[14] Febrile neutropenia remains a major cause of hospitalization apart from admissions for chemoradiation. A study from India on patterns of mortality in children with ALL identified sepsis (53.3%) and bleeding (15.7%) as the most common causes of mortality.[15] Another study of 214 children with ALL treated as per UKALL-XI protocol reported 31 deaths. Causes of nonrelapse deaths were sepsis 18, stroke 3, bleeding 1, tumor lysis syndrome 1, and graft versus host disease 1; sepsis accounted for more than 50% of deaths in this study.[16]
In this study, no patient developed any significant complication to sedation or anesthesia. Literature shows low risk of complications of about 1.3% in pediatric treatments under anesthesia/sedation (A/S) in the outpatient setting. Delivery of safe and effective anesthesia for pediatric oncology patients receiving radiation therapy presents several challenges. The anesthesia provider must continuously monitor the patient's cardiovascular and respiratory status from a remote location. In recent years, propofol has become the standard of anesthesia care for radiation therapy in children. Its merits for use in pediatric external-beam radiation therapy include rapid and predictable induction of anesthesia, easily titratable depth of anesthesia, maintenance of spontaneous ventilation with minimal need for airway manipulation, and rapid recovery.[17] Beyond age 3, the requirement for A/S decreases in an age-dependent fashion, with a small cadre of older children having difficulty enough with sustained immobilization that A/S is necessary. Data reveal that General anaesthesia (GA) use is necessary in all patients age 3 and younger, with an age-dependent decrease until age 13. About 10% of children 12 or older still require A/S.[18]
RT is known to cause a broad range of adverse effects that have the potential to impact numerous functional domains and quality of life. Cognitive dysfunction and endocrinopathy are considered the most prevalent sequelae of irradiation, whereas vasculopathy with stroke and malignant transformation are more severe but less common. Efforts to minimize sequelae, including the use of chemotherapy as the preferred initial treatment for younger patients, have been given priority in the design of treatment regimens for pediatric low-grade gliomas.[19] Increased conformality of RT could decrease the incidence and severity of late effects. The advent of fully computerized highly precise radiation delivery systems such as three-dimensional conformal RT, intensity-modulated RT, fractionated stereotactic RT, Image-guided RT, helical tomotherapy, etc., today enables the radiation oncologists to treat only the tumor-bearing area and spare the surrounding critical structures, thereby decreasing acute and delayed toxicities.[20] Proton therapy has shown encouraging results in the treatment of pediatric tumors because the unique physical dosimetry allows for improved dose distribution.[21,22] However, proton therapy is limited in use by its scarce availability and cost issues.
Children with cancer often experience significant levels of pain, and their pain is generally undermanaged. With the transition of care of cancer patients from the hospital to the home setting, parents are largely responsible for children's pain management. However, parents may have misconceptions of analgesic use, which can lead to under-treatment of pain in children. Understanding and addressing barriers to children's cancer pain management in the home setting will aid in alleviating unnecessary pain in this vulnerable patient population.[23,24] Caring for a child with cancer is a distressing experience, which can affect parents in the long-term. Beyond treatment termination, parents continue to be exposed to illness-related stressors such as uncertainty about cure/relapse, physical or emotional late effects, and risk of second cancer. Studies have described parents' difficult adjustment, particularly when their child had received intense treatments, such as in the care of brain tumor patients. Distress may be impairing for vulnerable parents and may impact a child's coping and adjustment. Moderate quality evidence and expert consensus informed a strong recommendation for parents and caregivers to receive early and on-going assessment of their mental health needs with access to appropriate interventions facilitated to optimize parent, child, and family well-being.[25,26]
Conclusion
With the increasing incidence of pediatric cancers, it is of paramount importance that these cases are timely diagnosed and adequately managed. Every patient needs individualized treatment rather than blindly following international guidelines. Pediatric oncology, as a discipline, is likely to improve in days to come and lots of steps need to be undertaken to improve the prognosis of these cases. Every patient should be offered adequate supportive care both in out- and in-door settings to improve their quality of life. A significant percentage of children experience emotional distress during and after therapy, hence there is a need for targeted early screening and psychosocial interventions to support family functioning and coping skills. A more comprehensive understanding of infertility after cancer is crucial for counseling and decision making about future conception attempts and fertility preservation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank all allied specialties of Oncology and the Pediatrics Department for their support in managing these cases and in carrying out the study.
References
- Arora B, Kanwar V. Childhood cancers in India: Burden, barriers, and breakthroughs. Indian J Cancer 2009;46:257-9.
- Ellison LF, Pogany L, Mery LS. Childhood and adolescent cancer survival: A period analysis of data from the Canadian Cancer Registry. Eur J Cancer 2007;43:1967-75.
- Magrath I, Steliarova-Foucher E, Epelman S, Ribeiro RC, Harif M, Li CK, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol 2013;14:e104-16.
- Swaminathan R, Sankaranarayanan R. Under-diagnosis and under-ascertainment of cases may be the reasons for low childhood cancer incidence in rural India. Cancer Epidemiol 2010;34:107-8.
- Arora PR, Misra R, Mehrotra S, Mittal C, Sharma S, Bagai P, et al. Pilot initiative in India to explore the gonadal function and fertility outcomes of a cohort of childhood cancer survivors. J Hum Reprod Sci 2016;9:90-3.
- Antal Z, Sklar CA. Gonadal function and fertility among survivors of childhood cancer. Endocrinol Metab Clin North Am 2015;44:739-49.
- Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: A review of population-based cancer registries. Indian Pediatr 2014;51:218-20.
- Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer 2009;46:264-73.
- Arora RS, Eden T, Pizer B. The problem of treatment abandonment in children from developing countries with cancer. Pediatr Blood Cancer 2007;49:941-6.
- Ramzan M, Yadav SP, Sachdeva A. Treatment abandonment is a major hurdle to improving survival in childhood cancer in the developing world. Pediatr Blood Cancer 2013;60:159-60.
- Yadav SP, Rastogi N, Kharya G, Misra R, Ramzan M, Katewa S, et al. Barriers to cure for children with cancer in India and strategies to improve outcomes: A report by the Indian Pediatric Hematology Oncology Group. Pediatr Hematol Oncol 2014;31:217-24.
- Bhat S, Yadav SP, Suri V, Patir R, Kurkure P, Kellie S, et al. Management of childhood brain tumors: Consensus report by the Pediatric Hematology Oncology (PHO) chapter of Indian Academy of Pediatrics (IAP). Indian J Pediatr 2011;78:1510-9.
- Arora B, Banavali SD. Pediatric oncology in India: Past, present and future. Indian J Med Paediatr Oncol 2009;30:121-3.
- Mendes AV, Sapolnik R, Mendonça N. New guidelines for the clinical management of febrile neutropenia and sepsis in pediatric oncology patients. J Pediatr (Rio J) 2007;83 2 Suppl: S54-63.
- Marwaha RK, Kulkarni KP, Bansal D, Trehan A. Pattern of mortality in childhood acute lymphoblastic leukemia: Experience from a single center in Northern India. J Pediatr Hematol Oncol 2010;32:366-9.
- Yadav SP, Dua V, Sachdeva A. Sepsis is a major barrier to improving survival in childhood acute lymphoblastic leukemia in the developing world. J Pediatr Hematol Oncol 2011;33:636.
- Anghelescu DL, Burgoyne LL, Liu W, Hankins GM, Cheng C, Beckham PA, et al. Safe anesthesia for radiotherapy in pediatric oncology: St. Jude Children's Research Hospital experience, 2004-2006. Int J Radiat Oncol Biol Phys 2008;71:491-7.
- McMullen KP, Hanson T, Bratton J, Johnstone PA. Parameters of anesthesia/sedation in children receiving radiotherapy. Radiat Oncol 2015;10:65.
- Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 2009;27:3691-7.
- Paulino AC, Mazloom A, Terashima K, Su J, Adesina AM, Okcu MF, et al. Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 2013;119:2654-9.
- McMullen KP, Kerstiens J, Johnstone PA. Practical aspects of pediatric proton radiation therapy. Cancer J 2014;20:393-6.
- Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys 2014;89:1060-8.
- Fortier MA, Wahi A, Bruce C, Maurer EL, Stevenson R. Pain management at home in children with cancer: A daily diary study. Pediatr Blood Cancer 2014;61:1029-33.
- Fortier MA, Wahi A, Maurer EL, Tan ET, Sender LS, Kain ZN. Attitudes regarding analgesic use and pain expression in parents of children with cancer. J Pediatr Hematol Oncol 2012;34:257-62.
- Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer 2015;62 Suppl 5:S632-83.
- Leclair T, Carret AS, Samson Y, Sultan S. Stability and repeatability of the distress thermometer (DT) and the Edmonton Symptom Assessment System-Revised (ESAS-r) with parents of childhood cancer survivors. PLoS One 2016;11:e0159773.
References
- Arora B, Kanwar V. Childhood cancers in India: Burden, barriers, and breakthroughs. Indian J Cancer 2009;46:257-9.
- Ellison LF, Pogany L, Mery LS. Childhood and adolescent cancer survival: A period analysis of data from the Canadian Cancer Registry. Eur J Cancer 2007;43:1967-75.
- Magrath I, Steliarova-Foucher E, Epelman S, Ribeiro RC, Harif M, Li CK, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol 2013;14:e104-16.
- Swaminathan R, Sankaranarayanan R. Under-diagnosis and under-ascertainment of cases may be the reasons for low childhood cancer incidence in rural India. Cancer Epidemiol 2010;34:107-8.
- Arora PR, Misra R, Mehrotra S, Mittal C, Sharma S, Bagai P, et al. Pilot initiative in India to explore the gonadal function and fertility outcomes of a cohort of childhood cancer survivors. J Hum Reprod Sci 2016;9:90-3.
- Antal Z, Sklar CA. Gonadal function and fertility among survivors of childhood cancer. Endocrinol Metab Clin North Am 2015;44:739-49.
- Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: A review of population-based cancer registries. Indian Pediatr 2014;51:218-20.
- Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer 2009;46:264-73.
- Arora RS, Eden T, Pizer B. The problem of treatment abandonment in children from developing countries with cancer. Pediatr Blood Cancer 2007;49:941-6.
- Ramzan M, Yadav SP, Sachdeva A. Treatment abandonment is a major hurdle to improving survival in childhood cancer in the developing world. Pediatr Blood Cancer 2013;60:159-60.
- Yadav SP, Rastogi N, Kharya G, Misra R, Ramzan M, Katewa S, et al. Barriers to cure for children with cancer in India and strategies to improve outcomes: A report by the Indian Pediatric Hematology Oncology Group. Pediatr Hematol Oncol 2014;31:217-24.
- Bhat S, Yadav SP, Suri V, Patir R, Kurkure P, Kellie S, et al. Management of childhood brain tumors: Consensus report by the Pediatric Hematology Oncology (PHO) chapter of Indian Academy of Pediatrics (IAP). Indian J Pediatr 2011;78:1510-9.
- Arora B, Banavali SD. Pediatric oncology in India: Past, present and future. Indian J Med Paediatr Oncol 2009;30:121-3.
- Mendes AV, Sapolnik R, Mendonça N. New guidelines for the clinical management of febrile neutropenia and sepsis in pediatric oncology patients. J Pediatr (Rio J) 2007;83 2 Suppl: S54-63.
- Marwaha RK, Kulkarni KP, Bansal D, Trehan A. Pattern of mortality in childhood acute lymphoblastic leukemia: Experience from a single center in Northern India. J Pediatr Hematol Oncol 2010;32:366-9.
- Yadav SP, Dua V, Sachdeva A. Sepsis is a major barrier to improving survival in childhood acute lymphoblastic leukemia in the developing world. J Pediatr Hematol Oncol 2011;33:636.
- Anghelescu DL, Burgoyne LL, Liu W, Hankins GM, Cheng C, Beckham PA, et al. Safe anesthesia for radiotherapy in pediatric oncology: St. Jude Children's Research Hospital experience, 2004-2006. Int J Radiat Oncol Biol Phys 2008;71:491-7.
- McMullen KP, Hanson T, Bratton J, Johnstone PA. Parameters of anesthesia/sedation in children receiving radiotherapy. Radiat Oncol 2015;10:65.
- Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 2009;27:3691-7.
- Paulino AC, Mazloom A, Terashima K, Su J, Adesina AM, Okcu MF, et al. Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 2013;119:2654-9.
- McMullen KP, Kerstiens J, Johnstone PA. Practical aspects of pediatric proton radiation therapy. Cancer J 2014;20:393-6.
- Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys 2014;89:1060-8.
- Fortier MA, Wahi A, Bruce C, Maurer EL, Stevenson R. Pain management at home in children with cancer: A daily diary study. Pediatr Blood Cancer 2014;61:1029-33.
- Fortier MA, Wahi A, Maurer EL, Tan ET, Sender LS, Kain ZN. Attitudes regarding analgesic use and pain expression in parents of children with cancer. J Pediatr Hematol Oncol 2012;34:257-62.
- Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer 2015;62 Suppl 5:S632-83.
- Leclair T, Carret AS, Samson Y, Sultan S. Stability and repeatability of the distress thermometer (DT) and the Edmonton Symptom Assessment System-Revised (ESAS-r) with parents of childhood cancer survivors. PLoS One 2016;11:e0159773.


 PDF
PDF  Views
Views  Share
Share

