Clinico-immunological response to intratumoral versus intravenous neoadjuvant chemotherapy in advanced pediatric solid malignancies
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(02): 80-84
DOI: DOI: 10.4103/0971-5851.116183
Abstract
Background: There is minimal literature on the use of intralesional chemotherapy in the pediatric age group. We undertook this present study to evaluate the two modalities (intratumoral and intravenous) of giving chemotherapy in terms of toxicity of chemotherapy, hematological parameters, efficacy of chemotherapy in reduction in volume of the tumor as well as resectability of tumor with special emphasis on immunological parameters. Materials and Methods: Advanced cases of Wilms′ tumor and Neuroblastoma were included in the study. Intratumoral chemotherapy was given through 25 G spinal needle under aseptic precautions and ultrasound guidance in the same dose as in systemic chemotherapy. Intravenous group was given chemotherapy in the usual way. Reassessment was carried out after every course of chemotherapy. Results: Group A included 16 cases of Wilms′ tumor and 6 cases of neuroblastoma. In group B, there were 14 cases of Wilms′ tumor and 8 of neuroblastoma. Vomiting, diarrhea, mucositis, and thrombophlebitis were more common in the intravenous group (P < 0.05). The fall in Immunoglobulin A, Immunogloblulin G, Immunoglobulin M, and T-cell rosetting was more common in the intravenous group (P < 0.05). Seventy percent of patients had completely resectable tumor at the end of 6 doses of intratumoral chemotherapy as compared to 50% resectability in the intravenous group (P < 0.05). Conclusion: Intratumoral chemotherapy, besides causing less of the adverse effects and increasing the resecability rate, also causes less suppression of the immune system. This may be offered as an alternative safe and effective modality of treatment for advanced solid tumors.
Keywords
Chemotherapy - intratumoral chemotherapy - intravenous chemotherapy - pediatric solid tumorsPublication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
There is minimal literature on the use of intralesional chemotherapy in the pediatric age group. We undertook this present study to evaluate the two modalities (intratumoral and intravenous) of giving chemotherapy in terms of toxicity of chemotherapy, hematological parameters, efficacy of chemotherapy in reduction in volume of the tumor as well as resectability of tumor with special emphasis on immunological parameters.
Materials and Methods:
Advanced cases of Wilms’ tumor and Neuroblastoma were included in the study. Intratumoral chemotherapy was given through 25 G spinal needle under aseptic precautions and ultrasound guidance in the same dose as in systemic chemotherapy. Intravenous group was given chemotherapy in the usual way. Reassessment was carried out after every course of chemotherapy.
Results:
Group A included 16 cases of Wilms’ tumor and 6 cases of neuroblastoma. In group B, there were 14 cases of Wilms’ tumor and 8 of neuroblastoma. Vomiting, diarrhea, mucositis, and thrombophlebitis were more common in the intravenous group (P < 0.05). The fall in Immunoglobulin A, Immunogloblulin G, Immunoglobulin M, and T-cell rosetting was more common in the intravenous group (P < 0.05). Seventy percent of patients had completely resectable tumor at the end of 6 doses of intratumoral chemotherapy as compared to 50% resectability in the intravenous group (P < 0.05).
Conclusion:
Intratumoral chemotherapy, besides causing less of the adverse effects and increasing the resecability rate, also causes less suppression of the immune system. This may be offered as an alternative safe and effective modality of treatment for advanced solid tumors.
INTRODUCTION
Pediatric malignancies are only second to trauma as the leading cause of morbidity and mortality in developed countries.[1] With an increasing control of infections, the malignant diseases are fast catching up to take the lead in developing countries.[2] In a developed country, the percentage of patients with an advanced disease (Stage III and IV) is 30-35%; in our country, it is 65-70%.[3] This poses a challenge to our health-care system, as these advanced cases invariably are candidates for neo-adjuvant chemotherapy.
To increase tolerance and to reduce the toxicity of chemotherapy, several authors have demonstrated that intra-arterial and intra-peritoneal modes of giving anticancer drugs are more effective than conventional intravenous mode.[4,5,6,7]
There is minimal literature on the use of intralesional chemotherapy in the pediatric age group. We undertook this present study to evaluate the two modalities (intratumoral and intravenous) of giving chemotherapy in terms of toxicity of chemotherapy, hematological parameters, efficacy of chemotherapy in reduction in volume of the tumor as well as resectability of tumor with the special emphasis on immunological parameters.
MATERIALS AND METHODS
This prospective study was conducted in the Department of Pediatric Surgery of the University Hospital from July 2004 to June 2010. It was approved by the Institute ethical committee. The parents were informed about the study, and written consent was taken from them. The study included patients with advanced (stage III and IV) Neuroblastoma or Wilms’ tumor.
Advanced cases of Wilms’ tumor and Neuroblastoma, proven by Fine needle aspiration cytology, which were not amenable to primary surgery and fresh cases with no previous treatment, were included in the study. The non-feasibility of surgical resection was decided by two senior consultants. The patients were allocated to 2 groups on a random basis: Study group (Group A) receiving intratumoral chemotherapy and Control group (Group B) receiving intravenous chemotherapy. Forty four children were included in the study. We had 22 patients in each group, of which group A included 16 cases of Wilms’ tumor and 6 cases of neuroblastoma. In group B, there were 14 cases of Wilms’ tumor and 8 of neuroblastoma. The age and sex was comparable in both groups. Patients were randomized on the basis of a random number table using the Strata-9 software (44 random numbers from 1 to 44 without replacement were randomized into two groups, or blocks, on the basis of the random number table).
The neo-adjuvant chemotherapy regimen followed was Vincristine, Adriamycin, Actinomycin D Vincristine, Adriamycin, Actinomycin regime containing Vincristine 1.5 mg/m2 weekly for 6 weeks, Adriamycin 50 mg/m2 (on day1 in group A and in 3rd week in group B) and D actinomycin 45 μg/kg (on day 1 in group A in 3 divided doses, day 1-3 in group B).
Intravenous group was given chemotherapy in the usual way by a peripheral venous access.
Procedure
Intratumoral chemotherapy was given through 25 G spinal needle under aseptic precautions and ultrasound (USG) guidance in the same dose as in systemic chemotherapy. Injection hyaluronidase was given along with the chemotherapeutic agent in a study group to enhance the local distribution of the drug in tumor mass. Supportive therapy in the form of whole blood, platelets concentrate, and fresh frozen plasma was given as and when required.
Reassessment was carried out after every course of chemotherapy. The following parameters were studied: clinical toxicity, hematological parameter, immunological parameter, efficacy in terms of volume reduction, and resectability of tumor.
Clinical Toxicity was recorded in terms of nausea, vomiting, diarrhea, alopecia, fever, mucositis, pain, phlebitis, and skin necrosis.
Hematological parameters were recorded in terms of hemoglobin, total leucocyte count and platelet count.
Immunological parameters were recorded as level of IgG, IgA, IgM, and T-cell rosetting.
Efficacy of Treatment was recorded as a reduction of volume and resectability of tumor. Reduction of volume was assessed clinically and by USG (Volume of the tumor = 0.523 × Product of all dimensions of the tumor). The volume reduction was categorized as >50% size reduction, 25-50% size reduction and < 25% size reduction for easy comparison between the two groups, i.e., systemic and intratumoral group.
The grading of toxicity was in accordance with National Cancer Institute-common toxicity criteria-version 2.
For hematological parameters, grading was carried out in accordance to the Common toxicity criteria-2; given by the National institute of clinical excellence.
The method used to the detect immunoglobulins was Single Radial Immunodiffusion. This method was described by Fahey in 1968.
Detection of Viable T-Cells was determined by the percentage of T-cells that form rosettes with sheep erythrocytes. A true rosette comprised three or more sheep Red blood cells RBCs, clustered around a lymphocyte.
The pre-chemotherapy value of IgG, IgM, IgA, and T-cell rosette was taken as 100%. The weekly assessment was carried out. The value obtained was than compared to the pre-chemotherapy value to calculate a fall in percentage from the pre-chemotherapy value.
The data obtained for each group was statistically analyzed by Chi-square test, Fisher's exact test, and Student T-test. P value < 0.05 was taken as significant.
RESULTS
All patients in the intratumoral group completed the 6 doses of chemotherapy in 6 weeks, except two patients, who expired after 1st week. The cause was unknown and probably they died due to advanced disease process. In intravenous group, 4 children expired and 2 were lost to follow-up. Hence, there were 20 patients in group A and 16 in group B.
Initially, no patient had vomiting. By 6th week, 2/20 (9%) had grade 2 nausea and 2/20 (9%) had grade 3 nausea in intratumoral group whereas 10/16 (62.5%) had grade 2 nausea and 2/16 (12.5%) had grade 3 nausea in the intravenous group (P < 0.05).
There was no patient had diarrhea at the time of presentation. By 6th week, in intratumoral group, 2/20 (10%) had grade 1 diarrhea, and 2/20 (10%), had grade 2 diarrhea, whereas in the intravenous group, 8/16 (50%), had grade 2 diarrhea (P < 0.05).
By 6th week, in intratumoral group, 2/20 (10%) had grade 2 mucositis, whereas in intravenous group, 4/8 (50%) had grade 1 mucositis, and 3/8 (37.5%) had grade 2 mucositis, and 1/8 (12.5%) had grade 3 mucositis (P < 0.01).
By 3rd week, in intravenous group, all children had thrombophlebitis, and it was difficult to cannulate them. Only 1 child in the intratumoral group developed phlebitis in the 5th week (P < 0.001). No child in the intratumoral group had skin necrosis whereas in the intravenous group, 6/16 (37.5%) had necrosis (P < 0.05). Pain was experienced more by all patients receiving intratumoral chemotherapy, and the difference was statistically significant. Regarding the total leukocyte or platelet count, the difference was not statistically significant (P = NS).
The fall in IgA, from pre-chemotherapy values, was higher for intravenous group as compared to intratumoral group. By the completion of 6 cycles of chemotherapy, serum IgA had fallen from 100% to 52.58% of its pre-chemotherapy value in intratumoral group, whereas in the intravenous group it had fallen from 100% to 38.98% of its pre-chemotherapy value (P < 0.01) [Figure 1].
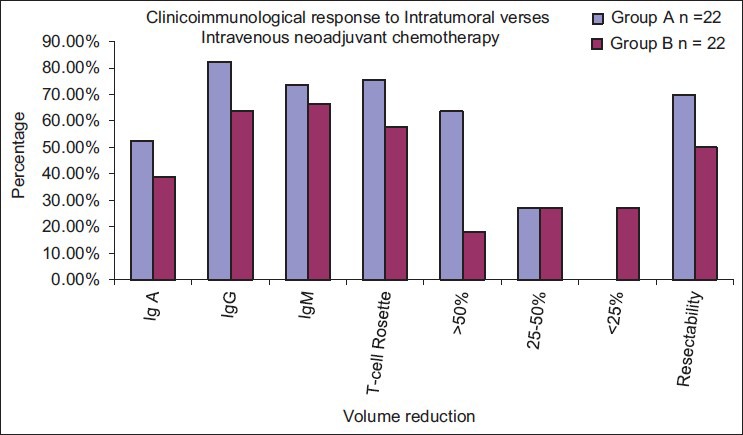
| Fig. 1 Graphical comparison of the two treatment modalities. Immunosuppression is less in intratumoral chemotherapy
The fall in IgG, from pre-chemotherapy values, were higher for intravenous group as compared to intratumoral group. By the completion of 6 cycles of chemotherapy, serum IgG had fallen from 100% to 82.39% of its pre-chemotherapy value in intratumoral group, whereas in the intravenous group it had fallen from 100% to 63.91% of its pre-chemotherapy value (P < 0.001).
The fall in IgM, from pre-chemotherapy values, was higher for intravenous group as compared to intratumoral group. By the completion of 6 cycles of chemotherapy, serum IgM had fallen from 100% to 73.54% of its pre-chemotherapy value in intratumoral group whereas in the intravenous group it had fallen from 100% to 66.47% of its pre-chemotherapy value. The fall was significant in the first 4 weeks; maximum in the 1st week.
The fall in T-cell rosette from pre-chemotherapy values, were higher for intravenous group as compared to intratumoral group. By the completion of 6 cycles of chemotherapy, T-cell rosetting capacity had fallen from 100% to 75.36% of its pre-chemotherapy value in intratumoral group, whereas in the intravenous group it had fallen from 100% to 57.86% of its pre-chemotherapy value (P < 0.001).
At the completion of 6 cycles of chemotherapy, more than 50% reduction in tumor volume was attained in 14/22 (63.64%) of patients in the intratumoral group, as compared to 4/22 (18.18%) in the intravenous group (P < 0.05) [Figure 2].
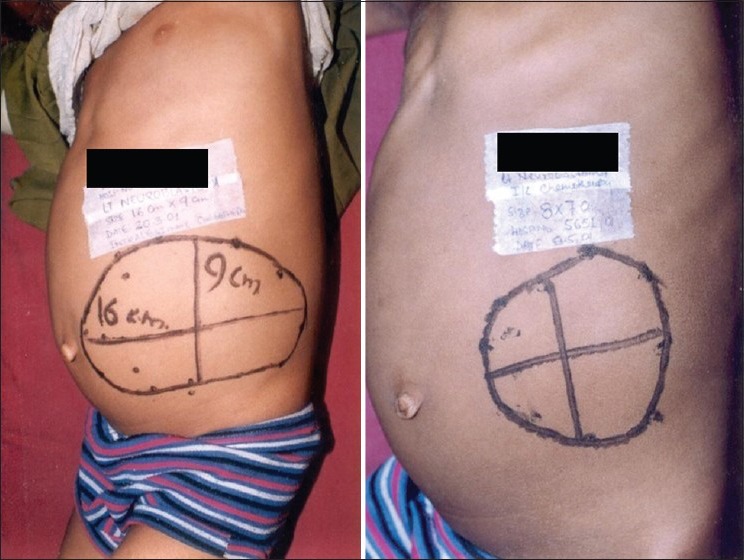
| Fig. 2 Results both before and after the administration of intratumoral chemotherapy. There is a visible reduction in size of the lump
70% of patients had completely resectable tumor at the end of 6 doses of intratumoral chemotherapy as compared to 50% resectability in the intravenous group. This difference in resectability was statistically significant.
DISCUSSION
In spite of advances in cancer research, advanced solid pediatric malignancies are difficult to manage mainly because of the advanced disease process, leading to poor general condition, and intolerance to multimodal therapy.
Wilms’ tumor is a common solid malignant neoplasm of childhood.[8] It is used as a model disease in Pediatric Oncology for multimodality treatment,[9] which is standardized by the Wilms’ tumor study group. Chemotherapy with actinomycin D was begun in 1954 and vincristine was added in 1963.[10] The survival, which was less than 50% in 1950's is now approaching 80-90%.[11] Vincristine and D actinomycin do not need hepatic metabolization for their activation. However, clearance is done by the liver. Hence, dose alteration may be needed in hepatic derangement.[12,13] Adriamycin is metabolized to adriamycinol by the liver.[14] In patients with normal hepatic function, Adriamycin has been found to be the principal agent, which is responsible for therapeutic effect. However, in patients with liver disease, adriamycinol tends to be more important, as it has been reported that total adriamycinol plasma concentrations are higher than total Adriamycin plasma concentrations.[14]
The response to conventional intravenous chemotherapy in advanced disease is varied due to the systemic spread of drugs and poor tolerance of the already malnourished patient to these highly toxic drugs leading to postponement of chemotherapy in between. This has led to further extensive search for an alternative route of chemotherapy in advanced pediatric solid malignancies (e.g., Wilms’ tumor and neuroblastoma) to improve tolerance and response rate. Intraarterial or intraperitoneal chemotherapy have better response, along with less complications, but they are more expensive, require better skill and adequate set up. There have been only sporadic studies regarding intratumoral injection of chemotherapeutic drugs in the pediatric age group.[15]
The International Society of Pediatric Oncology (SIOP) has promoted the use of preoperative treatment of children with Wilms’ tumor with chemotherapy, without histologic confirmation of the diagnosis before therapy is initiated. By following this protocol, a decrease in rupture rate from 33% to 4% and a lower surgical complication rate has been reported.[16] The pre-operative treatment may significantly decrease the apparent stage of the children's disease.[17]
In has been showed that pre-operative chemotherapy is useful in patients who represent a particularly high-risk for surgical intervention owing to a very large locally invasive primary tumor, extensive metastatic disease, massive ascitis, or unstable metabolic status.[18,19,20] However, the toxicity of conventional systemic chemotherapy affords limited effectiveness and frequently compromises the quality of life for patients. In this context, it should be noticed that targeted or localized drug delivery should be the major goal of chemotherapy.[21] Despite the wide-spread use of chemotherapy, there is only limited clinical use of intratumoral chemotherapy for even those cancers, which have well-defined primary lesions.[21]
Studies have shown that there is stimulation of tumor-specific systemic immune response, which eradicates metastasis. It results from processing of tumor specific antigen expressed by the tumor cell debris in immune competent individuals following intratumoral chemotherapy.[21] The present study suggests that intratumoral chemotherapy causes less suppression of the immune system as compared to the intravenous chemotherapy. As no other study has been carried out, until now in this regard, it is not possible to have a comparative evaluation of the same. The effect of tumor spillage is not of much concern in an advanced stage; however, it was not seen in this study.
There is a limitation to this study. We have only compared the regression in the tumor size and immunological response on the basis of therapy, which was the basis of this study. The overall effect on survival and follow-up was not a part of this study. This has been assessed in other studies by our group, which shows a favorable outcome to this modality of treatment.[15,22]
To conclude, intratumoral chemotherapy, besides causing less of the adverse effects and increasing the resecability rate, also causes less suppression of the immune system. This may be offered as an alternative safe and effective modality of treatment for advanced solid tumors. However, its mechanism of action and release in the systemic circulation needs to be evaluated in future study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 Graphical comparison of the two treatment modalities. Immunosuppression is less in intratumoral chemotherapy

| Fig. 2 Results both before and after the administration of intratumoral chemotherapy. There is a visible reduction in size of the lump


 PDF
PDF  Views
Views  Share
Share

