Clinicopathological Correlation of Adenoid Cystic Carcinoma: A Notorious Masquerader and Clinical Paradox
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(03): 276-281
DOI: DOI: 10.4103/ijmpo.ijmpo_141_16
Abstract
Background: Adenoid cystic carcinoma (ACC) is an uncommon tumor with nonspecific clinicoradiological features thereby masquerading other nonneoplastic and neoplastic entities. Materials and Methods: Cases of ACC were retrospectively reviewed over a period of 4 years. The clinical details of these patients including fine-needle aspiration cytology (FNAC) and imaging findings were retrieved. Diagnosis was confirmed on histomorphology and supplemented with immunohistochemistry (IHC). Results:: Thirty cases of ACC were included in the study. Mean patient age was 55.5 years with a slight female preponderance. Among the 30 ACCs, 10 (33.4%) were located in submandibular gland, 7 (23.4%) in parotid gland, 6 (20%) in sublingual gland, 2 (6.7%) in lung and one each (3.33%) in nasal cavity, breast, cervix, lip, and skin of face. Preoperative imaging was suggestive of malignancy in 29 cases while a single case of parotid gland ACC was misdiagnosed as benign salivary gland neoplasm. FNAC was performed in 29 cases with a diagnostic accuracy of 82.7%. Histopathological examination showed characteristic features of ACC in all cases with perineural invasion seen in 7 cases. On IHC, positivity for cytokeratin was seen in all cases, cluster of differentiation 117 in 24 cases, thyroid transcription factor-1 in two cases and human epidermal growth factor receptor/neu in two cases. All cases were negative for estrogen receptor and progesterone receptor IHC. Mean Ki-67 score was 47.8%. Conclusion: ACCs are notorious tumors showing slow growth kinetics with propensity for perineural invasion, late recurrences, and distant metastasis. It should be kept in mind as a differential diagnosis at unusual sites other than salivary glands.
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Adenoid cystic carcinoma (ACC) is an uncommon tumor with nonspecific clinicoradiological features thereby masquerading other nonneoplastic and neoplastic entities. Materials and Methods: Cases of ACC were retrospectively reviewed over a period of 4 years. The clinical details of these patients including fine-needle aspiration cytology (FNAC) and imaging findings were retrieved. Diagnosis was confirmed on histomorphology and supplemented with immunohistochemistry (IHC). Results:: Thirty cases of ACC were included in the study. Mean patient age was 55.5 years with a slight female preponderance. Among the 30 ACCs, 10 (33.4%) were located in submandibular gland, 7 (23.4%) in parotid gland, 6 (20%) in sublingual gland, 2 (6.7%) in lung and one each (3.33%) in nasal cavity, breast, cervix, lip, and skin of face. Preoperative imaging was suggestive of malignancy in 29 cases while a single case of parotid gland ACC was misdiagnosed as benign salivary gland neoplasm. FNAC was performed in 29 cases with a diagnostic accuracy of 82.7%. Histopathological examination showed characteristic features of ACC in all cases with perineural invasion seen in 7 cases. On IHC, positivity for cytokeratin was seen in all cases, cluster of differentiation 117 in 24 cases, thyroid transcription factor-1 in two cases and human epidermal growth factor receptor/neu in two cases. All cases were negative for estrogen receptor and progesterone receptor IHC. Mean Ki-67 score was 47.8%. Conclusion: ACCs are notorious tumors showing slow growth kinetics with propensity for perineural invasion, late recurrences, and distant metastasis. It should be kept in mind as a differential diagnosis at unusual sites other than salivary glands.
Introduction
Adenoid cystic carcinoma (ACC) is a relatively uncommon tumor, with a reported incidence of 4.5 cases per million individuals.[1] It most commonly involves the salivary glands.[2] Involvement of lacrimal gland, breasts, uterine cervix, esophagus, lungs, and prostate is exceedingly rare and infrequently reported.[3]
Owing to nonspecific clinicoradiological features, these tumors masquerade other nonneoplastic and neoplastic entities. On literature search scattered case reports and case series of site specific ACCs were found without any large study on the incidence, distribution and clinicopathological features of ACC at different sites. Keeping in view these considerations, we retrospectively analyzed cases of ACCs over a period of 4 years in a tertiary care setting. Our study highlights the clinicopathological features of ACC with special emphasis on the utility of preoperative diagnostic modalities such as imaging and fine-needle aspiration cytology (FNAC).
Materials and Methods
The archives of Department of Histopathology were retrospectively reviewed from January 2012 to January 2016. Of the 57,890 cases received, cases of ACC were included in the study. The medical records and clinical details of these patients including preoperative FNAC and/or radiological findings on computed tomography (CT)/magnetic resonance imaging (MRI)/mammography were retrieved. FNAC was performed wherever possible. Repeat aspiration was performed if the first FNA attempt yielded inadequate material or was inconclusive for diagnosis. Smears were then air dried and stained with May-Grünwald Giemsa stain. All the patients underwent curative surgery. The excised specimen was fixed in 10% neutral buffered formalin and sent for histopathological examination. Diagnosis was confirmed on hematoxylin and eosin (H and E) stained, formalin-fixed paraffin embedded sections. These tumors were graded as per recommendations by Szanto et al.[4] Tumors containing only tubular or cribriform growth pattern were categorized as Grade I tumors, tumors containing cribriform or tubular growth with <30>30% solid component as Grade III tumors. A detailed immunohistochemical (IHC) panel consisting of cytokeratin (CK) (Biocare Medical, Clone: AE1/AE3), cluster of differentiation 117 (CD117) 117 (Biocare Medical, Clone: EP10), thyroid transcription factor-1 (TTF-1) (Biocare Medical, Clone: 8G7G3/1), estrogen receptor (ER) (Thermo Scientific, Clone: SP1), progesterone receptor (PR) (Thermo Scientific, Clone: SP2), human epidermal growth factor receptor (HER2/neu) (BioGenex, Clone EP1045Y), and Ki-67 (Thermo Scientific, Clone: SP6) was applied to all cases. In brief, sections measuring 3–4 μm thick were cut, deparaffinized with xylene and brought to water through graded levels of alcohol. Endogenous peroxidase activity was blocked by treating the slides with hydrogen peroxide for 30 min at room temperature. Antigen retrieval was done by immersing the slides in citrate buffer using the pressure cooker method. Then, the slides were incubated overnight with the primary antibody (rabbit polyclonal) at 4°C in a humidified chamber. The following day secondary antibody was added. The sections were then incubated with di amino benzidine for visualization of the peroxidase reaction. After being washed in water, the sections were counter stained with hematoxylin, dehydrated in alcohol, cleared in xylene, and mounted. IHC was interpreted in a binary fashion as positive or negative. Ki-67 index was calculated by counting the total number of Ki-67 positive cells in 100 consecutive tumor cells.
The FNAC smears, H and E stained sections, and IHC slides of all the cases were reviewed by two pathologists and correlated with the clinical findings including radiology.
Results
Out of 57,890 cases received in the department over a period of 4 years, we found only 30 cases of ACC [Table 1]. The mean patient age at the time of surgery was 55.5 years (range 19–64 years). There were 13 male and 17 female patients with a male:female ratio of 1:1.3. Among these 30 tumors, 10 (33.4%) were located in the submandibular gland, 7 (23.4%) in the parotid gland, 6 (20%) in the sublingual gland, 2 (6.7%) in the lung and one each (3.33%) in nasal cavity, breast, cervix, lip, and skin of face.
|
Site |
Number of cases (%) |
|---|---|
|
Submandibular gland |
10 (33.4) |
|
Parotid gland |
7 (23.4) |
|
Sublingual gland |
6 (20) |
|
Lung |
2 (6.7) |
|
Breast |
1 (3.33) |
|
Cervix |
1 (3.33) |
|
Nasal cavity |
1 (3.33) |
|
Lip |
1 (3.33) |
|
Cutaneous (face) |
1 (3.33) |
|
Total |
30 |
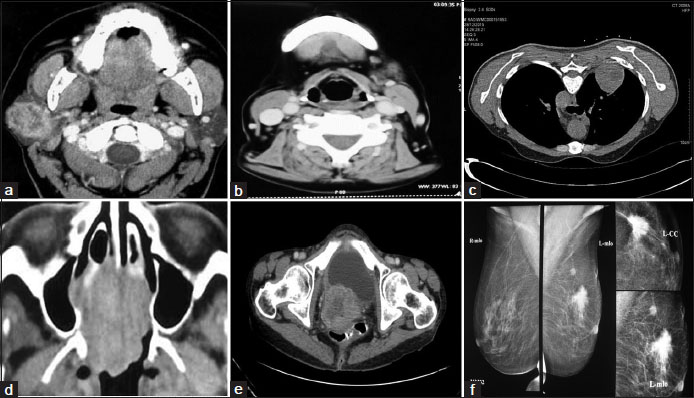
| Figure.1:Contrast enhanced computed tomography image shows (a) Heterogeneously enhancing mass lesion involving superficial and deep lobes of right parotid gland, (b) Small lobulated mass lesion just anterior to the left submandibular gland with areas of necrosis, (c) Heterogeneously enhancing mass in posterior segment of right upper lobe, (d) Homogeneous mass involving the nasal septum along with bony erosion of medial wall of right orbit, (e) Heterogeneously enhancing cervical mass seen obstructing the endocervical canal, (f) Breast mammography image shows a spiculated mass in upper outer quadrant of left breast suggestive of BIRADS IV
FNAC was performed in all cases except for the mass in uterine cervix due to inaccessibility of lesion. Further, CT-guided FNAC was performed for the lung lesions. FNA smears were moderately cellular with cells arranged in variably sized cohesive clusters and occasional cup shaped fragments [Figure 2]a. The individual tumor cells were small with scant basophilic cytoplasm and central round hyperchromatic nucleus. Numerous variable sized, spherical, and pink hyaline globules were seen both within and outside the cell clusters [Figure 2]b. Accurate diagnosis of ACC was made in 82.7

| Figure.1:Contrast enhanced computed tomography image shows (a) Heterogeneously enhancing mass lesion involving superficial and deep lobes of right parotid gland, (b) Small lobulated mass lesion just anterior to the left submandibular gland with areas of necrosis, (c) Heterogeneously enhancing mass in posterior segment of right upper lobe, (d) Homogeneous mass involving the nasal septum along with bony erosion of medial wall of right orbit, (e) Heterogeneously enhancing cervical mass seen obstructing the endocervical canal, (f) Breast mammography image shows a spiculated mass in upper outer quadrant of left breast suggestive of BIRADS IV
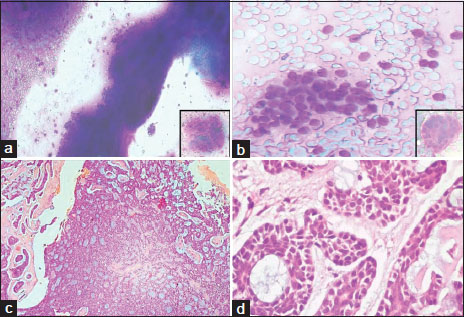
| Figure.2Photomicrograph of May Grünwald Giemsa stained fine needle aspiration smears show; (a) Moderate cellularity with small basaloid cells arranged in cohesive clusters and occasional cup shaped fragments (inset) (×200). (b) Tumor cells are embedded within spherical, pink hyaline globules (inset) (×200). Photomicrograph of H and E stained sections show; (c) Cribriform arrangement of tumor cells forming pseudocysts filled with basement membrane like basophilic material (×100). (d) Tubular arrangement of cells which are round to cuboidal with scant eosinophilic cytoplasm and central hyperchromatic nucleus (×400)
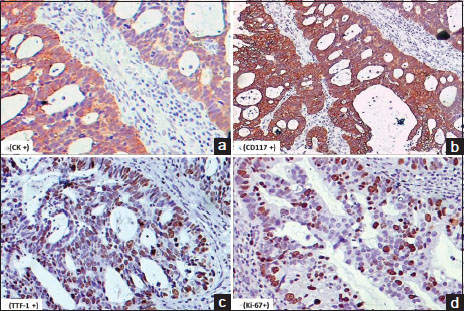
| Figure.3On immunohistochemistry tumor cells were positive for; (a) cytokeratin (immunostain, ×200), (b) cluster of differentiation 117 (immunostain, ×200), (c) thyroid transcription factor-1 (immunostain, ×200), (d) Ki-67 (immunostain, ×200)
References
- Bonaparte JP, Hart R, Trites J, Taylor MS. Incidence of adenoid cystic carcinoma in nova scotia: 30-year population-based epidemiologic study. J Otolaryngol Head Neck Surg 2008; 37: 642-8
- Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck – A 20 years experience. Int J Oral Maxillofac Surg 2004; 33: 25-31
- Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: A review of chemotherapy and molecular therapies. Oral Oncol 2006; 42: 759-69
- Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984; 54: 1062-9
- Spies JW. Adenoid cystic carcinoma. Arch Surg 1930;21:365-404.
- Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med 2011; 135: 511-5
- Chummun S, McLean NR, Kelly CG, Dawes PJ, Meikle D, Fellows D, Fellows S. et al. Adenoid cystic carcinoma of the head and neck. Br J Plast Surg 2001; 54: 476-80
- Muslimani AA, Ahluwalia MS, Clark CT, Daw HA. Primary adenoid cystic carcinoma of the breast: Case report and review of the literature. Int Semin Surg Oncol 2006; 3: 17
- Muslimani AA, Ahluwalia MS, Clark CT, Daw HA. Primary adenoid cystic carcinoma of the breast: Case report and review of the literature. Int Semin Surg Oncol 2006;3:17.
- Chin S, Kim Z. Adenoid cystic carcinoma of the breast: Report of two cases with immunohistochemical profile of C-kit and MYB overexpression. Indian J Pathol Microbiol 2014; 57: 611-3
- Li N, Xu L, Zhao H, El-Naggar AK, Sturgis EM. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer 2012; 118: 3945-53
- Maheshwari GK, Dave KS, Wadhwa MK, Gopal U, Shah R. Adenoid cystic carcinoma of the uterine cervix with pulmonary metastasis 11 years after radiotherapy: Case report. Turk J Cancer 2000; 30: 181-5
- Grayson W, Taylor LF, Cooper K. Adenoid cystic and adenoid basal carcinoma of the uterine cervix: Comparative morphologic, mucin, and immunohistochemical profile of two rare neoplasms of putative 'reserve cell' origin. Am J Surg Pathol 1999; 23: 448-58
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995; 75: 191-202
- Kumari S, Saugat R, Kapoor A, Paramanandhan M, Soni G, Sirohi P. Adenoid cysticcarcinoma of lung: A very unusual presentation. Int J Sci Rep 2015; 1: 143-5
- Bhalara RV, Gamit MJ, Popat M, Gandhi SH, Dhruva GA. Cytomorphology of primary adenoid cystic carcinoma of lung: An exceedingly rare case. APALM 2015; 2: 223-6
- Batra R, Belaldavar BP. Adenoid cystic carcinoma of the nasal septum: A rare case report. J Sci Soc 2013; 40: 39-40
- Belaldavar BP, Batra R. Adenoid cystic carcinoma of the nasal septum: A rare case report. J Sci Soc 2013;40:39-40.
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol 2015; 8: 251-5
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007; 12: 20-37
- Mehdi I, Shah AH, Moona MS, Verma K, Abussa A, Elramih R, et al. Synchronous and metachronous malignant tumours expect the unexpected. J Pak Med Assoc 2010;60:905-9.
- Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Management of adenoid cystic carcinoma of minor salivary glands. J Oral Maxillofac Surg 2006; 64: 1114-20
- Norberg-Spaak L, Dardick I, Ledin T. Adenoid cystic carcinoma: Use of cell proliferation, BCL-2 expression, histologic grade, and clinical stage as predictors of clinical outcome. Head Neck 2000; 22: 489-97
- Dy GK, Miller AA, Mandrekar SJ, Aubry MC, Langdon RMJr, Morton RF. Dy GK, Miller AA, Mandrekar SJ, Aubry MC, Langdon RM Jr, Morton RF, et al A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: A CALGB and NCCTG study. Ann Oncol 2005; 16: 1811-6
- ;Dori S, Vered M, David R, Buchner A. HER2/neu expression in adenoid cystic carcinoma of salivary gland origin: An immunohistochemical study. J Oral Pathol Med 2002; 31: 463-7
- ;An J, Park S, Sung SH, Cho MS, Kim SC. Unusual expression of thyroid transcription factor 1 and napsin a in metastatic adenoid cystic carcinoma of extrapulmonary origin in the lung. Am J Clin Pathol 2014; 141: 721-7
- Giannini PJ, Shetty KV, Horan SL, Reid WD, Litchmore LL. Adenoid cystic carcinoma of the buccal vestibule: A case report and review of the literature. Oral Oncol 2006; 42: 1029-32
- Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg 1992; 164: 623-8
- Rapidis AD, Givalos N, Gakiopoulou H, Faratzis G, Stavrianos SD, Vilos GA. et al. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol 2005; 41: 328-35
- da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, de Almeida OP, Kowalski LP. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol 2006; 42: 139-46
- Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D'Amico F. et al. Risk factors for local recurrence of adenoid cystic carcinoma: The role of postoperative radiation therapy. Am J Otolaryngol 1999; 20: 281-6
- Pinakapani R, Nallan C, Reddy L, Srujana Y, Mamatha B, Waghray S. Adenoid cystic carcinoma of the head and neck – Literature review. Qual Prim Care 2015; 23: 309-14


 PDF
PDF  Views
Views  Share
Share

