Clinicopathological profile of gastrointestinal lymphomas in Kashmir
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 251-255
DOI: DOI: 10.4103/0971-5851.195736
Abstract
Background: The histological categorization of lymphoma has been a source of controversy for many years for both clinicians and pathologists. Clinicopathologic information of gastrointestinal lymphomas in Indian subcontinent is lacking. We studied histopathological spectrum of Primary Gastrointestinal Lymphomas (PGIL) and attempted to classify the G.I. lymphomas based on the recent WHO classification in to major histological types and immunological categories. Material and Methods: This study was done to evaluate the clinicopathological pattern of 100 cases with a histopathological diagnosis of primary gastrointestinal lymphoma at a tertiary care hospital. All patients of primary gastrointestinal lymphomas were included with the help of medical records over a 11-years period that is, January 2005 to December 2015. Results: The study included 100 cases (60 males, 40 females; mean age 51.43 years; age range 4.5-90 years) . The disease involved stomach in 82 (82%), small intestine in 8 (8%), large bowel and rectum in 8 (8%), gall bladder in 1 (1%) and oesophagus in 1 (1%). 82 (82%) of the 100 cases were Diffuse Large B cell lymphomas; 12 (12%) were Extra Nodal Marginal Zone Lymphomas (ENMZL of MALT type) 2 (2%) IPSID 2 (2%) of Mantle cell lymphoma morphology, 1 (1%) Burkitt's and 1(1%) enteropathy associated T cell lymphoma. The commonest presenting symptom was abdominal pain. 99 (99%) of 100 tumours were classified as B-cell lymphomas immunohistochemically and majority exhibited monoclonal light chain restriction on kappa/lambda staining. In addition; Burkitt's lymphoma showed positivity for CD 10. One tumour (1%) showed positivity for T-cell markers. The data demonstrated that primary GI NHL is more common among males, mainly in their fifth decade. Abdominal pain is the most common presenting symptom, with stomach being the most commonly involved site. Diffuse large cell lymphoma is the most frequent histologic subtype, followed by extranodal marginal-zone B cell lymphoma (MALT type). H. Pylori infection was observed in cases with low grade MALT lymphomas. Striking was the observation of two cases of IPSID (a disease commonly found in Mediterranean countries) and one case of enteropathy associated T cell lymphoma. Conclusion: EGD, imaging, light microscopic examination and immunohistochemical workup for B and T cell markers and staining for light chains to assist documentation of monoclonality are of precise diagnostic value in gastrointestinal lymphomas and form a part of the diagnostic workup.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The histological categorization of lymphoma has been a source of controversy for many years for both clinicians and pathologists. Clinicopathologic information of gastrointestinal lymphomas in Indian subcontinent is lacking. We studied histopathological spectrum of Primary Gastrointestinal Lymphomas (PGIL) and attempted to classify the G.I. lymphomas based on the recent WHO classification in to major histological types and immunological categories.
Material and Methods:
This study was done to evaluate the clinicopathological pattern of 100 cases with a histopathological diagnosis of primary gastrointestinal lymphoma at a tertiary care hospital. All patients of primary gastrointestinal lymphomas were included with the help of medical records over a 11-years period that is, January 2005 to December 2015.
Results:
The study included 100 cases (60 males, 40 females; mean age 51.43 years; age range 4.5-90 years). The disease involved stomach in 82 (82%), small intestine in 8 (8%), large bowel and rectum in 8 (8%), gall bladder in 1 (1%) and oesophagus in 1 (1%). 82 (82%) of the 100 cases were Diffuse Large B cell lymphomas; 12 (12%) were Extra Nodal Marginal Zone Lymphomas (ENMZL of MALT type) 2 (2%) IPSID 2 (2%) of Mantle cell lymphoma morphology, 1 (1%) Burkitt's and 1(1%) enteropathy associated T cell lymphoma. The commonest presenting symptom was abdominal pain. 99 (99%) of 100 tumours were classified as B-cell lymphomas immunohistochemically and majority exhibited monoclonal light chain restriction on kappa/lambda staining. In addition; Burkitt's lymphoma showed positivity for CD 10. One tumour (1%) showed positivity for T-cell markers. The data demonstrated that primary GI NHL is more common among males, mainly in their fifth decade. Abdominal pain is the most common presenting symptom, with stomach being the most commonly involved site. Diffuse large cell lymphoma is the most frequent histologic subtype, followed by extranodal marginal-zone B cell lymphoma (MALT type). H. Pylori infection was observed in cases with low grade MALT lymphomas. Striking was the observation of two cases of IPSID (a disease commonly found in Mediterranean countries) and one case of enteropathy associated T cell lymphoma.
Conclusion:
EGD, imaging, light microscopic examination and immunohistochemical workup for B and T cell markers and staining for light chains to assist documentation of monoclonality are of precise diagnostic value in gastrointestinal lymphomas and form a part of the diagnostic workup.
INTRODUCTION
Primary gastrointestinal lymphomas (PGILs) can be defined as “A lymphoma that has presented with the main bulk of disease in the gastrointestinal (G.I.) tract, with or without the involvement of the contiguous lymph nodes, necessitating treatment of that site.”[1]
The histological categorization of lymphoma has been a source of controversy for many years for both clinicians and pathologists. Many competing lymphoma classifications were in use. The Revised European-American lymphoma (REAL) classification was proposed by International Lymphoma study group in April 1993, to classify lymphoid neoplasms.[2] The new WHO classification[3] is merely an update of the REAL classification, with some minor modifications. Controversy currently exists as to whether the spectrum of lymphomas found in the gut can be currently accommodated within the conventional classification or whether a site-specific classification of G.I. lymphoma is needed.[4]
Low B grade gastric lymphomas are associated with Helicobacter Pylori infection. It has been suggested that stomach does not possess organized lymphoid tissue, mucosa-associated lymphoid tissue (MALT) appears in response to infection by H. Pylori.[5,6] It has been further shown that cellular proliferation in low-grade B-cell lymphoma is stimulated by an appropriate strain of H. Pylori.[7]
Lack of adequate clinicopathological information in the Indian subcontinent and in view of the above facts, we studied the histopathological spectrum of G.I. lymphomas at Government Medical College, Srinagar. We attempted to classify the lymphomas based on the WHO classification into major histological types based on immunohistochemistry and also observed the association of H. Pylori with the lymphomas.
MATERIALS AND METHODS
We analyzed patient material diagnosed with PGIL at Government Medical College, Srinagar. The study period extended from January 2005 to December 2015. The search for cases was carried out using International Classification of Diseases codes for lymphomas and by a year wise search of records maintained at the clinic. The terms used to search cases included lymphoma, G.I. lymphomas, immunoproliferative small intestinal disease (IPSID), and extranodal lymphomas. The clinical details were included as per a pro forma which included name, hospital number, age, sex, clinical presentation, radiological diagnosis, esophagogastroduodenoscopy (EGD) findings, organs involved, operative findings, laboratory investigations, macroscopic features, primary microscopic diagnosis, extent of involvement, margins of resection, and regional lymph node involvement. Patients included in the study were only those with no enlargement of peripheral or mediastinal nodes, normal white cell count, predominance of alimentary tract lesions and those with no involvement of liver and spleen. The specimens preserved in 10% formalin were used to study the gross appearance of the tumors and further material obtained from specimens and processed as and when needed. Sections were obtained from tissue bits processed and embedded in paraffin and also from preserved tissue blocks. All cases were stained for H and E and immunohistochemical markers, according to manufacturer's instructions on 5 µ thick paraffin sections. The sections were stained for CD3, CD20, CD10, and Light immunoglobulin chain (λ/Κ) antigen. Slides were reviewed to confirm the diagnosis and all the material was classified based on the recent WHO classification for non-Hodgkin lymphoma (NHL) and modified site-specific classification was used to further categorize the lymphomas. Morphological features including lymphoepithelial lesions, tumor pattern, and tumor cell size were documented to give morphological subtype to the tumor. Any associated changes including type of inflammatory infiltrate in the surrounding mucosa and around the tumor was noted. H. Pylori were looked for in the surrounding mucosa, graded using Sydney system for gastritis (visual analog scale) in H and E stained and giemsa-stained sections.
RESULTS
The total number of cases with suspected gastrointestinal lymphomas over 11 year period extending from January 2005 to December 2015 was 102, out of which only 100 were confirmed with primary gastrointestinal involvement and the other two were found to have involvement of spleen and central nervous system. The series comprised of 100 cases which included 60 (60%) males and 40 (40%) females; with male: female ratio of 3:2. The lymphomas occurred predominantly in middle aged and elderly. There was a wide age distribution from 4.5-90 years and mean age being 51.43 years. The site specific distribution of 100 cases was; 82 (82%) stomach, 8 (8%) small intestine, 8 (8%) large bowel & rectum, 1 (1%) gall bladder & 1(1%) oesophagus. The presenting symptoms varied according to the site of involvement and in majority included abdominal pain, weight loss, vomiting (due to pyloric obstruction), dyspepsia, obstructive jaundice (due to spread of disease to lymph nodes in porta hepatitis), chronic diarrhoea and rectal bleeding. On H&E, 82 (82%) of the 100 cases were of Diffuse Large cell type; 12 (12%) were Extra Nodal Marginal Zone Lymphomas (ENMZL of MALT type); 2 (2%) IPSID (one with diffuse large cell transformation), 2 (2%) had Mantle cell lymphomas morphology (MCL), 1(1%) Burkitt's and 1 (1%) lymphoma of small bowel had anaplastic features and was associated with coeliac disease.
Gastric lymphomas revealed DLCL morphology in 70 (85.3%) cases [Figure [Figure1a1a–d] and ENMZL in 12 (14.6%) [Figure [Figure2a2a–b] cases. Small bowel lymphomas included 4(50%) DLCL, 2 (25%) IPSID, 1(12.5%) Burkitt's and 1 (12.5%) case with anaplastic morphology; Large bowel lymphomas had DLCL morphology in 6 (75%) and 2 (25%) exhibited mantle cell lymphoma morphology and tumours from gall bladder and oesophagus [Figure [Figure3a3a–c] revealed morphology of DLCL. All cases of gastric lymphomas showed H. Pylori in the surrounding mucosa (with density ranging from 3 to 4+).
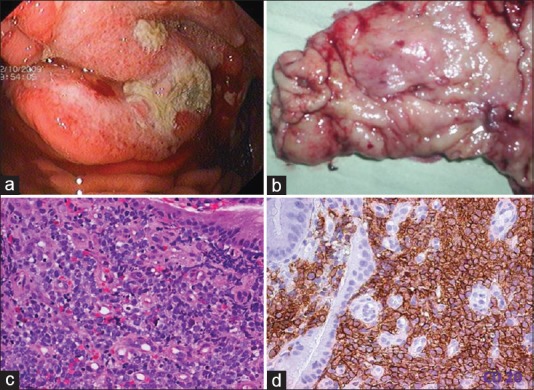
| Fig. 1 Diffuse large cell lymphoma stomach. (a) Upper gastrointestinal endoscopy image showing a gastric tumor with central ulceration. The tumor is bulging with increased vascularity. (b) Gross examination of the gastrectomy specimen revealed an ulceroproliferative tumor with central ulceration in the antrum. (c) Photomicrograph of the tumor. Monotonous population of lymphoid cells with moderate to abundant cytoplasm. The cells have hyperchromatic nuclei (H and E, ×400). (d) CD 20 diffuse membrane staining of lymphoid cells (IHC, ×400)
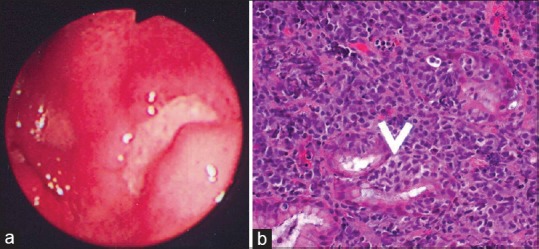
| Fig. 2 Extranodal marginal zone lymphoma mucosa-associated lymphoid tissue lymphoma. (a) Upper gastrointestinal endoscopy revealed an ulcerated growth in the body of the stomach. The ulcer base is covered with slough. (b) The presence of lymphoepithelial lesions. These lesions were identified by the unequivocal presence of invasion and partial destruction of gastric glands or crypts by tumor cell aggregates. Tumor cells were small to medium sized lymphocytes with irregularly shaped nuclei and moderately abundant cytoplasm (H and E, ×400)
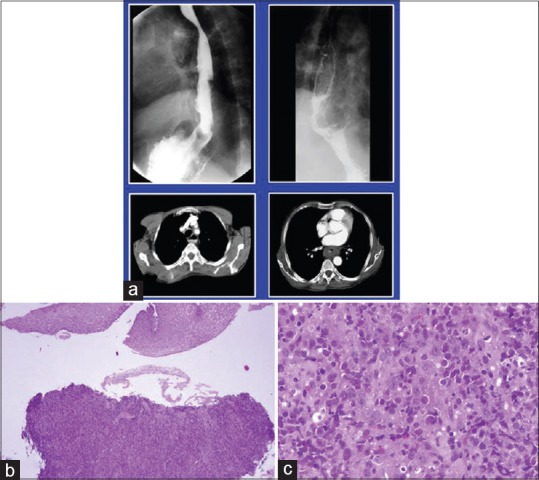
| Fig. 3 Diffuse large cell lymphoma esophagus. (a) Barium enema showing narrowing of upper esophagus. Lower window shows computed tomography scan of the same tumor. (b and c); biopsy from the growth reveals esophageal mucosa with a monotonous lymphoid tumor in the submucosal area morphologically consistent with diffuse large cell lymphoma (H and E, ×100, ×400)
The Immunohistochemical analysis revealed 99 (99%) of 100 tumours positive for B cell markers and 1 (1%) tumours in showed positivity for T cell markers. Almost all B cell positive tumours showed light chain restriction confirming monoclonal nature of these tumours.
The gross appearances of the specimens of gastrointestinal lymphomas were as follows: polypoid mass in 70 (70%), ulcerating tumour in 25 (25%), diffuse thickening in 1 (1%) and multiple polyps were identified in 3 (3%) specimens. One specimen (1%) revealed no identifiable tumour on gross and revealed only loss of mucosal rugosity. Microscopic examination of diffuse large cell lymphomas revealed diffuse effacement of the glandular architecture by a uniform population of cells. Lympho- epithelial lesions were identified in all cases. Neoplastic cells were predominantly of large size (size compared to histiocyte nucleus). Most of the cases showed predominantly centroblasts (large cleaved cells with round to oval vesicular nuclei & 2-3 small nucleoli seen closely opposed to the nuclear membrane and a narrow rim of cytoplasm) admixed with variable number of large centrocytes having cleaved nuclear contour and inconspicuous nucleoli. The marginal zone lymphomas (MALT) revealed the presence of lymphoepithelial lesions. These lesions were identified by unequivocal presence of invasion and partial destruction of gastric glands or crypts by tumor cell aggregates. Tumor cells were small to medium sized lymphocytes with irregularly shaped nuclei and moderately abundant cytoplasm. Burkitt's lymphoma Small bowel revealed tumour cells with abundant eosinophilic to clear cytoplasm, hyperchromatic nucleus with prominent nucleoli and Starry sky pattern of tumour as a result of scattered tingible body macrophages. The tumour cells show cytoplasmic positivity for CD10 immunostain confirming the histopathological diagnosis of Burkitt's lymphoma [Figure [Figure4a4a–b].
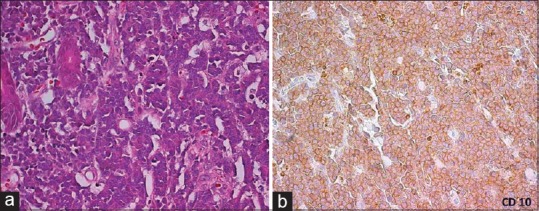
| Fig. 4 Burkitt's lymphoma small bowel (a). Tumor cells with abundant eosinophilic to clear cytoplasm. Hyperchromatic nucleus and prominent nucleoli. Starry sky pattern of tumor as a result of scattered tingible body macrophages (H and E, ×40). (b) The tumor cells show cytoplasmic positivity for CD10 immunostain confirming the histopathological diagnosis of Burkitt's lymphoma
Mantle cell lymphoma involved the mucosa and submucosa. The malignant cells had the appearance of small atypical lymphocytes. Most cases were composed of small to medium sized lymphoid cells with irregular nuclear contours, most closely resembling centrocytes. The nuclei had moderately dispersed chromatin but inconspicuous nucleoli [Figure [Figure5a5a–b]. Immunoproliferative small intestinal disease (IPSID) revealed a lymphoplasmacytic infiltrate confined to mucosa at places and was seen to diffusely infiltrate as well. The infiltrate was seen to obliterate the villous architecture. Lymphoid follicles and lymphoepithelial lesions were also identified. One of the two cases of IPSID showed transformation to a DLBCL. This was evidenced by the appearance in the tumour of large atypical cells. The T cell lymphoma of small bowel; on H&E examination revealed presence of large pleomorphic cells with anaplastic morphology. The surrounding small bowel mucosa showed evidence of celiac disease. This was evidenced by presence of intraepithelial lymphocytes [IEL's] (more than 50 IEL's per 100 nuclei) and marked villous atrophy. [Figure [Figure6a6a–b]. All the tumours were positive for B-cell markers except for one tumour which showed positivity for T cell markers. Monoclonality was established in majority of the cases with kappa and lambda immunostains. In addition; Burkitt's lymphoma showed surface/ membranous positivity for CD10 immunostain.
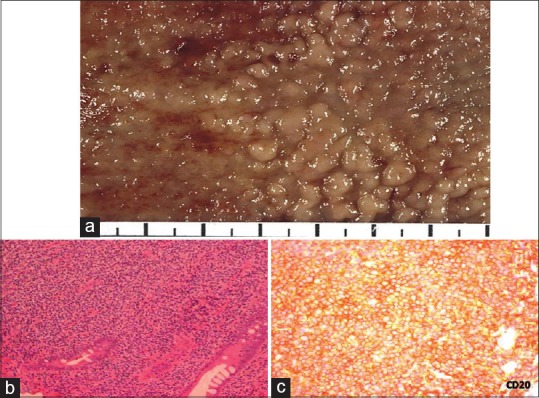
| Fig. 5 Mantle cell lymphoma large bowel. (a) Characteristic gross appearance of tumor exhibiting multiple polyps studding the large bowel mucosa 2–5 mm in size. (b) Mantle cell lymphoma involving mucosa and submucosa. Malignant cells have the appearance of small atypical lymphocytes. Tumor was composed of small to medium-sized lymphoid cells with irregular nuclear contours, most closely resembling centrocytes. The nuclei had moderately dispersed chromatin but inconspicuous nucleoli (H and E, ×200). (c) Tumor cells show cytoplasmic positivity for CD20 (CD20 immunostain; ×200)
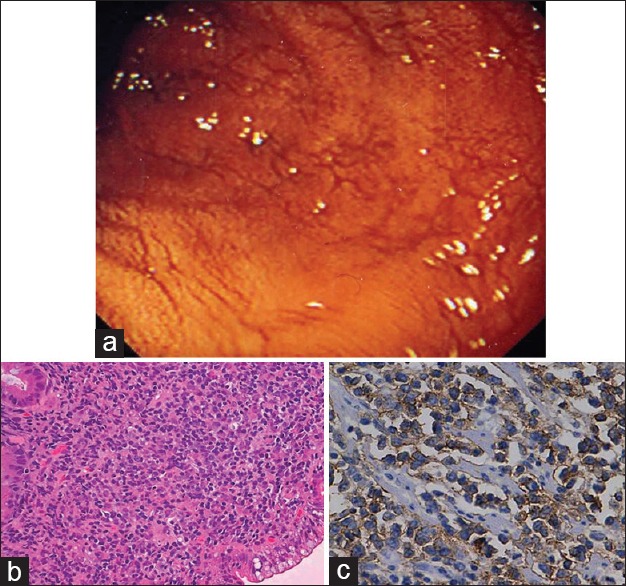
| Fig. 6 Enteropathy-associated T-cell lymphoma small bowel. (a) Endoscopic view of the small bowel mucosa showing flattened mucosa and prominent submucosal vessels. (b) The presence of large pleomorphic cells with scattered cells exhibiting anaplastic morphology. (c) Tumor cells show strong membranous positivity for CD3 in tumor cells (CD3 immunostain, ×400)
DISCUSSION
The G.I. tract is the most frequent site of extranodal involvement in NHL; however, primary G.I-NHL remains relatively rare.[8] In this study of fifty cases of G.I. lymphomas conducted over a period of 10 years, stomach was the most common site of involvement followed by large bowel and small bowel. In majority of the studies, as in this study, stomach was the most common site of involvement.[9,10,11,12] Berger et al.[10] reported 17 cases of gastric, five cases of intestinal and one case of cecal lymphoma. Clinical findings were comparable to other studies which included; age group 26–88 years,[10,13,14] M:F ratio was 3:2,[14] abdominal pain being commonest presenting symptom.[9]
DLCL was the most common histologic type in this study comparable to observations made by studies from India and other parts of the world.[15,16,17,18] The second common histologic type was ENMZL/MALT lymphoma.
Primary gastric lymphoma as in our study is the most common form in Western countries. However, in the Middle East and Mediterranean countries, primary intestinal lymphoma is the most common form accounting for 50% of extranodal and 75% of G.I. lymphoma.[19] This is due to predominance in these countries of the so-called Mediterranean lymphoma better known as IPSID or alpha heavy chain disease.[16] Apart from different geographic prevalence, small intestinal lymphomas are associated with specific socioeconomic patterns.[17] They occur predominantly in young adults and seem to be linked with gastroenteritis in childhood. The two cases of IPSID observed in our study were middle-aged and were known to have a low socioeconomic status.
Enteropathy-associated T-cell lymphomas (EATLs) are distinctive subtype of lymphomas arising from the T-cells found in the small intestinal mucosa.[20] This lymphoma is as a consequence of refractory/chronic untreated cases of coeliac disease and in genetically susceptible individuals. EATL is a rare lymphoma with global incidence of about 0.5–1/million.[21] Gluten sensitive individuals usually have a human leukocyte antigen-DQ isoform that appears to be responsible for EATL in the majority of the cases. These individuals present a proteolytically protected region of α2-gliadin to T-cells; resulting in constant over-stimulation of T-cells and neoplastic overgrowth.[22] The patient in our series had documented coeliac disease of long duration.
Primary NHL of the esophagus and gall bladder are exceedingly rare. A recent study[23] on gall bladder lymphomas found these tumors to be quite rare, patients presented with features of cholecystitis and the predominant histological subtype in their series is DLCL. Esophageal lymphomas are very rare; accounting for only 1% of all G.I. lymphomas. Primary lymphomas at this site are even rarer. In a series on esophageal lymphomas, only two of thirty-seven cases were found to be primary lymphomas; one a Hodgkin's lymphoma and another one NHL.[24]
This longitudinal study over 11-year period revealed that primary G.I. lymphomas are uncommon tumors seen in the pathological material available or received at our institute. Striking was the observation of occurrence of two cases of IPSID in our society which are mainly reported from the Mediterranean countries and also the observation of EATL which is a relatively rare entity reported from India.[25] The pattern of immunehistologic findings was also different in that almost all the cases in the present study were positive for B-cell markers, and only one case was T-cell positive.
CONCLUSION
EGD, imaging, light microscopic examination, and immunohistochemical workup for B- and T-cell markers and staining for light chains to assist documentation of monoclonality are of precise diagnostic value in G.I. lymphomas and form a part of the diagnostic workup.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Diffuse large cell lymphoma stomach. (a) Upper gastrointestinal endoscopy image showing a gastric tumor with central ulceration. The tumor is bulging with increased vascularity. (b) Gross examination of the gastrectomy specimen revealed an ulceroproliferative tumor with central ulceration in the antrum. (c) Photomicrograph of the tumor. Monotonous population of lymphoid cells with moderate to abundant cytoplasm. The cells have hyperchromatic nuclei (H and E, ×400). (d) CD 20 diffuse membrane staining of lymphoid cells (IHC, ×400)

| Fig. 2 Extranodal marginal zone lymphoma mucosa-associated lymphoid tissue lymphoma. (a) Upper gastrointestinal endoscopy revealed an ulcerated growth in the body of the stomach. The ulcer base is covered with slough. (b) The presence of lymphoepithelial lesions. These lesions were identified by the unequivocal presence of invasion and partial destruction of gastric glands or crypts by tumor cell aggregates. Tumor cells were small to medium sized lymphocytes with irregularly shaped nuclei and moderately abundant cytoplasm (H and E, ×400)

| Fig. 3 Diffuse large cell lymphoma esophagus. (a) Barium enema showing narrowing of upper esophagus. Lower window shows computed tomography scan of the same tumor. (b and c); biopsy from the growth reveals esophageal mucosa with a monotonous lymphoid tumor in the submucosal area morphologically consistent with diffuse large cell lymphoma (H and E, ×100, ×400)


 PDF
PDF  Views
Views  Share
Share

