Clinicopathological Study of 100 Cases of Neuroendocrine Neoplasms of the Gastroenteropancreatic System: A Tertiary Cancer Center Experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 340-344
DOI: DOI: 10.4103/ijmpo.ijmpo_217_18
Abstract
Background: The incidence of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) is on the rise. Although the clinicopathologic characteristics of NENs have been previously reviewed in the literature, the data published in the Indian literature so far are sparse. This study aims to review the clinicopathological features of GEP-NENs, diagnosed at our institution, and that were classified and graded according to the World Health Organization 2010 classification system. Materials and Methods: One hundred patients with GEP-NENs presenting to our institute from August 2012 to May 2016 were analyzed retrospectively. Demographic data and tumor characteristics were expressed as number, percentage, and mean value. Tumor grade was correlated to metastasis through the Chi-square test. p < 0.05 was considered statistically significant. Results: Of the 100 cases studied, 58 were male and 42 were female. The most common primary site was the pancreas (n = 36), followed by the small intestine (n = 19), esophagus (n = 17), stomach (n = 15), colon (n = 6), rectum (n = 4), and appendix (n = 3). The incidence of neuroendocrine tumor (NET) Grade 1 (NET G1) was higher (n = 40) compared to NET Grade 2 (NET G2) (n = 25) and neuroendocrine carcinoma Grade 3 (NEC G3) (n = 35). Overall in these 100 cases, NET G1 tumors and NET G2 tumors were most common in the pancreas (n = 18/36) and (n = 13/36), respectively. NEC G3 tumors were most common in the esophagus (n = 16/17). The most common site of distant metastasis was the liver (n = 23/26). Conclusion: We elucidated the epidemiological and clinicopathological features of patients presenting to our institute with GEP-NENs.
Keywords
Gastroenteropancreatic neuroendocrine neoplasm - neuroendocrine carcinoma Grade 3 - neuroendocrine tumor Grade 1 - neuroendocrine tumor Grade 2 - World Health Organization 2010 classificationPublication History
Received: 08 October 2018
Accepted: 10 November 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: The incidence of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) is on the rise. Although the clinicopathologic characteristics of NENs have been previously reviewed in the literature, the data published in the Indian literature so far are sparse. This study aims to review the clinicopathological features of GEP-NENs, diagnosed at our institution, and that were classified and graded according to the World Health Organization 2010 classification system. Materials and Methods: One hundred patients with GEP-NENs presenting to our institute from August 2012 to May 2016 were analyzed retrospectively. Demographic data and tumor characteristics were expressed as number, percentage, and mean value. Tumor grade was correlated to metastasis through the Chi-square test. p < 0.05 was considered statistically significant. Results: Of the 100 cases studied, 58 were male and 42 were female. The most common primary site was the pancreas (n = 36), followed by the small intestine (n = 19), esophagus (n = 17), stomach (n = 15), colon (n = 6), rectum (n = 4), and appendix (n = 3). The incidence of neuroendocrine tumor (NET) Grade 1 (NET G1) was higher (n = 40) compared to NET Grade 2 (NET G2) (n = 25) and neuroendocrine carcinoma Grade 3 (NEC G3) (n = 35). Overall in these 100 cases, NET G1 tumors and NET G2 tumors were most common in the pancreas (n = 18/36) and (n = 13/36), respectively. NEC G3 tumors were most common in the esophagus (n = 16/17). The most common site of distant metastasis was the liver (n = 23/26). Conclusion: We elucidated the epidemiological and clinicopathological features of patients presenting to our institute with GEP-NENs.
Keywords
Gastroenteropancreatic neuroendocrine neoplasm - neuroendocrine carcinoma Grade 3 - neuroendocrine tumor Grade 1 - neuroendocrine tumor Grade 2 - World Health Organization 2010 classificationIntroduction
Neuroendocrine neoplasms (NENs) originate from the neuroendocrine cell system distributed throughout the body. They can develop at any site, with the majority arising from the gastroenteropancreatic system (GEP). They comprise a heterogeneous family with complex clinical behavior.
The incidence of GEP-NENs was reported to be 3.65/100,000 individuals per year according to the surveillance, epidemiology, and end results database program.[1] The incidence has risen substantially over the past 30 years due to advanced diagnostic methods and increased awareness of the disorder.
In 1907, Oberndofer first described these tumors as “Carcinoid,” a carcinoma-like tumor which was considered to have less malignant potential.[2] In 2000 and 2004, respectively, the World Health Organization (WHO) classified neuroendocrine tumors (NETs) into well-differentiated tumors and poorly differentiated tumors.[3] According to the WHO 2010 classification, GEP-NENs are classified as NET and neuroendocrine carcinoma (NEC) based on cell proliferation. NETs are further subdivided into NET Grade 1 (NET G1) (mitoses <2/10 high-power field
In the WHO 2017 classification[5] and AJCC 8th edition, those tumors with typical morphology of well-differentiated tumors and with mitoses >20/10 HPF or Ki-67 index >20% are classified as “well-differentiated NET” but as Grade 3. This grading scheme (Grade 1–3) based on the above mitotic activity or Ki-67 index is recommended for well-differentiated GEP-NETs. In the present study, in addition to the demographic and clinical characteristics, we analyzed the histopathological feature along with the immunohistochemical (IHC) staining pattern of 100 cases of GEP-NENs presenting to our institute.
Materials and Methods
This was a hospital-based retrospective study of 100 cases of GEP-NENs diagnosed at our institution from August 2012 to May 2016. Patients with histopathological confirmation of the diagnosis of GEP-NENs from the primary site were included, whereas metastasis of unknown origin was excluded from the study. Clinicopathological characteristics, including age, gender, symptoms, primary location of the tumor, histopathological diagnosis, IHC findings, presence or absence of metastasis, and treatment given to the patients, were retrieved from the medical records. The tumor grade was determined according to the WHO 2010 classification, with the estimation of Ki-67 index in areas of high nuclear labeling (“Hotspots”). Mitoses/10 HPF were counted for grading in cases where Ki-67 was not available.
Biostatistics
The demographic data and tumor characteristics were expressed as number, percentage, and mean value. Tumor grade was correlated to metastasis using the Chi-square test. P < 0.05 was considered statistically significant.
Results
Clinical features
We identified a total of 100 patients diagnosed with GEP-NENs, of which 58 were male and 42 were female. The mean age at the diagnosis was 51.3 years (range: 22–77 years). The most common primary site was the pancreas (n = 36), followed by the small intestine (n = 19), esophagus (n = 17), stomach (n = 15), colon (n = 6), rectum (n = 4), and appendix (n = 3) [Figure 1]. In the pancreas, most of the NENs were localized in the head (n = 20), followed by tail and body. In the esophagus, most of the NENs were localized in the lower third (n = 11), followed by middle third (n = 3), and upper third (n = 3). Overall, the most frequent initial presentation was abdominal pain (n = 40), followed by dysphagia (n = 26), vomiting (n = 22), anorexia (n = 19), change in bowel habits (n = 17), weight loss (n = 11), gastrointestinal bleeding (n = 7), and jaundice (n = 7). Biopsies of the patients were received, on which the histopathological diagnoses were made, followed by the confirmation by immunohistochemistry.{Figure 1}
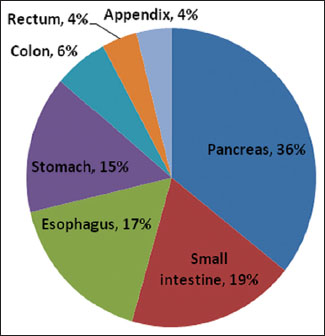
| Figure 1: Pie chart showing the distribution of primary tumor site
Histopathological characteristics
The growth patterns in NETs were either predominantly or a combination of nested, insular, glandular, trabecular, festoon, and gyriform (n = 65). NEC had a more diffuse growth pattern (n = 35) [Figure 2].{Figure 2}
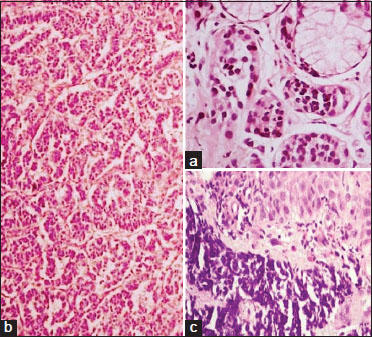
| Figure 2: (a) Nests of tumor cells of neuroendocrine tumor Grade 1 in the submucosa of the stomach (H and E, ×40). (b) Tumor cells of neuroendocrine tumor Grade 2 with a trabecular and gyriform pattern in the pancreas (H and E, ×10). (c) Tumor cells of neuroendocrine carcinoma Grade 3 with the submucosa in the esophagus (H and E, ×40)
Of the total 100 patients, 40 patients had NET G1 (40%), 25 patients had NET G2 (25%), and 35 patients had NEC G3 (35%). Overall, NET G1 and NET G2 were most common in the pancreas (n = 18/36) and (n = 13/36), respectively. Other common sites of NET G1 tumors were the small intestine, stomach, and colon, whereas the other common sites for NET G2 tumors were the stomach. The incidence of NEC G3 was the highest in the esophagus (n = 16/35) followed by the small intestine (n = 7/35). All the NEC G3 cases in our study were poorly differentiated NEC. The distribution of GEP-NENs according to site and grade is summarized in [Table 1].
Distribution of gastroenteropancreatic neuroendocrine neoplasms according to site and grade
|
Site |
NET G1, n (%) |
NET G2, n (%) |
NET G3, n (%) |
Total |
|---|---|---|---|---|
|
NET – Neuroendocrine tumor; NEC – Neuroendocrine carcinoma |
||||
|
Esophagus |
0 |
1 (5.8) |
16 (94.2) |
17 |
|
Stomach |
5 (33.3) |
8(53.3) |
2 (13.3) |
15 |
|
Small intestine |
10 (52.6) |
2 (10.6) |
7 (36.8) |
19 |
|
Appendix |
3 (100) |
0 |
0 |
3 |
|
Colon |
4 (66.7) |
0 |
2 (33.3) |
6 |
|
Rectum |
0 |
1 (25) |
3 (75) |
4 |
|
Pancreas |
18 (50) |
13 (36.1) |
5 (13.9) |
36 |
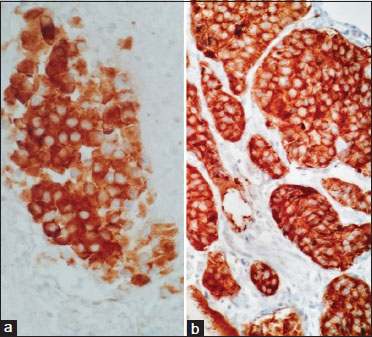
| Figure 3: (a) Immunohistochemical cytoplasmic positivity for synaptophysin (×40). (b) Immunohistochemical cytoplasmic positivity for chromogranin (×40)
Ki67 labelling index was helpful in grading of the tumours [Figure 4] in 85% (n = 85/100) of the cases, with the rest of 15% (n = 15/100) graded by mictotic count. Ki67 labelling index was in range of 0-2% with mean of 1% in NET G1, in range of 3-17% with mean of 8% in NET G2 and in range of 23-100% with mean of 62% in NEC G3.
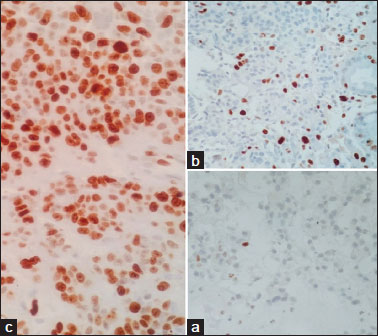
| Figure 4: (a) Ki 67 labeling index – 1% in neuroendocrine tumor Grade 1 (×40). (b) Ki 67 labeling index – 15% in neuroendocrine tumor Grade 2 (c) Ki 67 labeling index – 80% in neuroendocrine carcinoma Grade 3
Twenty-six patients developed distant metastasis (26%). The most common site of distant metastasis was the liver (88.4%, n = 23/26), followed by the lung, lymph nodes, and adrenal gland (3.9%, n = 1/26 in each of the 3 latter sites, respectively). Distant metastasis was more commonly seen in patients with NEC G3 tumors (n = 14) as compared with patients with NET G1 (n = 2) and NET G2 (n = 10) tumors, respectively. A statistically significant correlation was established between higher tumor grade and distant metastasis [Table 2]. Of the 14 Grade 3 tumors that metastasized, 6 were from the esophagus, 4 from the pancreas, 2 from the small intestine, and 2 from the rectum. Of the 10 Grade 2 tumors that metastasized, 5 were from the stomach, 3 from the pancreas, 1 from the rectum, and 1 from the esophagus. Of the 2 Grade 1 tumors that metastasized, 1 was from the pancreas and 1 from the colon.
Table 2 Relationship between tumor grade and metastasis
Treatment modalities
Forty-three (n = 43) patients underwent surgical resection, namely Whipple's procedure (n = 13), right hemicolectomy (n = 5), esophagectomy (n = 5), pancreatic resection (n = 3), appendectomy (n = 2), partial gastrectomy (n = 3), abdominoperineal resection (n = 2), and wide local excision (n = 10). Combination platinum-based chemotherapy regimens comprising etoposide and carboplatin/cisplatin were given to thirty (n = 30) patients. Six (n = 6) patients received radiotherapy and 17 (n = 17) patients received octreotide. Of thirty patients receiving chemotherapy, 14 (n = 14) patients received chemotherapy as the only treatment, 15 (n = 15) patients received chemotherapy adjuvant to surgery, and a single (n = 1) patient received chemotherapy with radiotherapy. Of six patients receiving radiotherapy, three patients (n = 3) received only palliative radiotherapy, two patients (n = 2) received radiotherapy adjuvant to surgery and chemotherapy, and single patient (n = 1) received radiotherapy with chemotherapy. Of the 17 patients who received octreotide, five patients (n = 5) were treated only with octreotide and 12 patients (n = 12) received octreotide after surgery.
Discussion
The incidence of NENs is on the rise. However, there have been limited data published on GEP-NENs in Indian literature. In this study, we analyzed data from 100 patients with primary GEP-NENs, classified according to the WHO 2010 classification system[4] and assessed the epidemiological and tumor characteristics.
In our study, the mean age at the diagnosis was 51 years, which was similar to various other studies.[6],[7],[8],[9] As others have previously reported,[6],[7] most patients in our study presented clinically with abdominal pain.
The small intestine and appendix have been cited as the most common site for GEP-NENs in the older literature,[1],[10] including studies in the United States[11],[12] and Norway.[13] In our study, the pancreas was the most common primary site for NENs, similar to other studies in the Chinese population.[7],[10],[14] This disparity in distribution is unclear, and racial or ethnic differences could be the possible cause. Better imaging techniques, including the use of endoscopic ultrasound-guided fine-needle aspiration and biopsy, make the tumors in the pancreas more accessible these days. The low detection rate of small intestinal NENs in our study could possibly be explained by the fact that most small intestinal NENs are asymptomatic and present with symptoms only after metastasis to the liver. Moreover, tumors in the small intestine are often small and difficult to access. The tumors may be submucosal and not easily visualized on routine endoscopy.
In our study, the incidence of NET G1 was higher than NET G2 and NEC G3, similar to other studies.[6],[7],[12],[15] When comparing tumor grade with site, overall NET G1 and G2 tumors were most commonly encountered in the pancreas. A similar Indian study[16] found majority of their tumors in the pancreas to be NET G1 (81.1%). In our study, most of the NENs in the esophagus were NEC G3, in accordance with other studies.[17],[18]
With regard to immunohistochemistry, the most commonly used markers to identify NENs are chromogranin A and synaptophysin.[6],[7],[15] Immunoreactivity to chromogranin A is more commonly seen in well-differentiated NETs, whereas synaptophysin is expressed in both well-differentiated NET and poorly differentiated NECs.[19] In our study, immunoreactivity to chromogranin and synaptophysin was found to be 95% each for Grade 1 tumors, 96% and 100% reactivity for Grade 2 tumors, with 80% and 94% reactivity for Grade 3 tumors, respectively. The liver was the most common site of distant metastasis in our study, similar to other studies.[6],[7],[15],[20] Predominantly, Grade 3 tumors were the ones to metastasize, followed by Grade 2 tumors.
The first choice of treatment for NETs is surgery, even if there are nodal or distant metastases. Whenever possible, the primary tumor should be removed, lymph nodes dissected, and distant metastasis excised.[21] In our study, 43 patients underwent surgical resection. In case of poorly differentiated, advanced GEP-NENs, chemotherapy is usually the first treatment option. In our study, the most widely used chemotherapy regimen was etoposide-cisplatin/etoposide-carboplatin, similar to another study[22] where cisplatin and etoposide were the most widely used chemotherapeutic drugs.
The new WHO 2017 classification, introduces a “Neuroendocrine Tumor Grade 3” category (NET G3), to recognize these better behaving, well-differentiated Grade 3 tumors and distinguish them from the poorly differentiated neuroendocrine carcinomas (NEC G3).[5] All the Grade 3 tumors (n = 35) identified from our study were poorly differentiated.
Conclusion
In our study, the pancreas was the most common site of GEP-NENs followed by the small intestine. Majority of the tumors in our study were NET G1 (40%). Most of the NETs Grade 1 and 2 were present in the pancreas, whereas most NEC G3 occurred in the esophagus. NEC G3 tumors were associated with distant metastasis more frequently as compared to NET G1 and NET G2 tumors, respectively. A national database of GEP-NENs should be established for studying these tumors. We believe that collecting regular national data and long-term follow-up can help in understanding the clinicopathological features of these tumors better.
Conflict of Interest
There are no conflicts of interest.
References
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063-72
- Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: Origins and perspectives of carcinoid tumors. Hum Pathol 2004; 35: 1440-51
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: The WHO classification. Ann N Y Acad Sci 2004; 2014: 13-27
- Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klopell G. et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman TF, Carneiro F, Hruban RH, Theise ND. editors WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010. 4. 13-4
- Lloyd RV, Osamura R, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon: IARC Press; 2017; 2017. 4.
- Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P, Sarin YK. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: A retrospective study. BMC Endocr Disord 2014; 14: 54
- Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord 2012; 12: 30
- Guo LJ, Wang CH, Tang CW. Epidemiological features of gastroenteropancreatic neuroendocrine tumors in Chengdu city with a population of 14 million based on data from a single institution. Asia Pac J Clin Oncol 2016; 12: 284-8
- Lim CH, Lee IS, Jun BY, Kim JS, Cho YK, Park JM. et al. Incidence and clinical characteristics of gastroenteropancreatic neuroendocrine tumor in Korea: A single-center experience. Korean J Intern Med 2017; 32: 452-8
- Chan DT, Luk AO, So WY, Kong AP, Chow FC, Ma RC. et al. Natural history and outcome in Chinese patients with gastroenteropancreatic neuroendocrine tumours: A 17-year retrospective analysis. BMC Endocr Disord 2016; 16: 12
- Strosberg J, Nasir A, Coppola D, Wick M, Kvols L, Sarin YK. et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol 2009; 40: 1262-8
- Alexiev BA, Drachenberg CB, Papadimitriou JC. Endocrine tumors of the gastrointestinal tract and pancreas: Grading, tumor size and proliferation index do not predict malignant behavior. Diagn Pathol 2007; 2: 28
- Sandvik OM, Søreide K, Gudlaugsson E, Kvaløy JT, Søreide JA. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br J Surg 2016; 103: 226-32
- Fan JH, Zhang YQ, Shi SS, Chen YJ, Yuan XH, Jiang LM. et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in China. Oncotarget 2017; 8: 71699-708
- Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Sinha S, Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM. et al Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: Multicenter study. Cancer Res Treat 2012; 44: 157-65
- Uppin MS, Uppin SG, Sunil CS, Hui M, Paul TH, Bheerappa N. et al. Clinicopathologic study of neuroendocrine tumors of gastroenteropancreatic tract: A single institutional experience. J Gastrointest Oncol 2017; 8: 139-47
- Capella C, Solcia E, Sobin LH, Arnold R. Endocrine tumours of the oesophagus. In: Hamilton SR, Aaltonen LA. editors World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC; 2000. p. 26-7
- Arnold R, Capella C, Klimstra DS, Kloppel G, Komminoth P. et al. Neuroendocrine neoplasms of the oesophagus. In: Bosman TF, Carneiro F, Hruban RH, Theise ND. editors WHO Classification of Tumours of the Digestive system. Lyon: IARC; 2000. p. 26-7
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol 2003; 14: 293-301
- Lombard-Bohas C, Mitry E, O'Toole D, Louvet C, Pillon D, Cadiot G, Sarin YK. et al. Thirteen-month registration of patients with gastroenteropancreatic endocrine tumours in France. Neuroendocrinology 2009; 89: 217-22
- Falconi M, Bettini R, Scarpa A, Capelli P, Pederzoli P. Surgical strategy in the treatment of gastrointestinal neuroendocrine tumours. Ann Oncol 2001; 12 Suppl 2: S101-3
- Zhang M, Zhao P, Shi X, Zhao A, Zhang L, Zhou L. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: A large, retrospective single-centre study. BMC Endocr Disord 2017; 17: 39
Address for correspondence
Publication History
Received: 08 October 2018
Accepted: 10 November 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Pie chart showing the distribution of primary tumor site

| Figure 2: (a) Nests of tumor cells of neuroendocrine tumor Grade 1 in the submucosa of the stomach (H and E, ×40). (b) Tumor cells of neuroendocrine tumor Grade 2 with a trabecular and gyriform pattern in the pancreas (H and E, ×10). (c) Tumor cells of neuroendocrine carcinoma Grade 3 with the submucosa in the esophagus (H and E, ×40)

| Figure 3: (a) Immunohistochemical cytoplasmic positivity for synaptophysin (×40). (b) Immunohistochemical cytoplasmic positivity for chromogranin (×40)

| Figure 4: (a) Ki 67 labeling index – 1% in neuroendocrine tumor Grade 1 (×40). (b) Ki 67 labeling index – 15% in neuroendocrine tumor Grade 2 (c) Ki 67 labeling index – 80% in neuroendocrine carcinoma Grade 3
- 1 Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063-72
- 2 Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: Origins and perspectives of carcinoid tumors. Hum Pathol 2004; 35: 1440-51
- 3 Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: The WHO classification. Ann N Y Acad Sci 2004; 2014: 13-27
- 4 Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klopell G. et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman TF, Carneiro F, Hruban RH, Theise ND. editors WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010. 4. 13-4
- 5 Lloyd RV, Osamura R, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon: IARC Press; 2017; 2017. 4.
- 6 Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P, Sarin YK. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: A retrospective study. BMC Endocr Disord 2014; 14: 54
- 7 Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord 2012; 12: 30
- 8 Guo LJ, Wang CH, Tang CW. Epidemiological features of gastroenteropancreatic neuroendocrine tumors in Chengdu city with a population of 14 million based on data from a single institution. Asia Pac J Clin Oncol 2016; 12: 284-8
- 9 Lim CH, Lee IS, Jun BY, Kim JS, Cho YK, Park JM. et al. Incidence and clinical characteristics of gastroenteropancreatic neuroendocrine tumor in Korea: A single-center experience. Korean J Intern Med 2017; 32: 452-8
- 10 Chan DT, Luk AO, So WY, Kong AP, Chow FC, Ma RC. et al. Natural history and outcome in Chinese patients with gastroenteropancreatic neuroendocrine tumours: A 17-year retrospective analysis. BMC Endocr Disord 2016; 16: 12
- 11 Strosberg J, Nasir A, Coppola D, Wick M, Kvols L, Sarin YK. et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol 2009; 40: 1262-8
- 12 Alexiev BA, Drachenberg CB, Papadimitriou JC. Endocrine tumors of the gastrointestinal tract and pancreas: Grading, tumor size and proliferation index do not predict malignant behavior. Diagn Pathol 2007; 2: 28
- 13 Sandvik OM, Søreide K, Gudlaugsson E, Kvaløy JT, Søreide JA. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br J Surg 2016; 103: 226-32
- 14 Fan JH, Zhang YQ, Shi SS, Chen YJ, Yuan XH, Jiang LM. et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in China. Oncotarget 2017; 8: 71699-708
- 15 Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Sinha S, Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM. et al Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: Multicenter study. Cancer Res Treat 2012; 44: 157-65
- 16 Uppin MS, Uppin SG, Sunil CS, Hui M, Paul TH, Bheerappa N. et al. Clinicopathologic study of neuroendocrine tumors of gastroenteropancreatic tract: A single institutional experience. J Gastrointest Oncol 2017; 8: 139-47
- 17 Capella C, Solcia E, Sobin LH, Arnold R. Endocrine tumours of the oesophagus. In: Hamilton SR, Aaltonen LA. editors World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC; 2000. p. 26-7
- 18 Arnold R, Capella C, Klimstra DS, Kloppel G, Komminoth P. et al. Neuroendocrine neoplasms of the oesophagus. In: Bosman TF, Carneiro F, Hruban RH, Theise ND. editors WHO Classification of Tumours of the Digestive system. Lyon: IARC; 2000. p. 26-7
- 19 Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol 2003; 14: 293-301
- 20 Lombard-Bohas C, Mitry E, O'Toole D, Louvet C, Pillon D, Cadiot G, Sarin YK. et al. Thirteen-month registration of patients with gastroenteropancreatic endocrine tumours in France. Neuroendocrinology 2009; 89: 217-22
- 21 Falconi M, Bettini R, Scarpa A, Capelli P, Pederzoli P. Surgical strategy in the treatment of gastrointestinal neuroendocrine tumours. Ann Oncol 2001; 12 Suppl 2: S101-3
- 22 Zhang M, Zhao P, Shi X, Zhao A, Zhang L, Zhou L. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: A large, retrospective single-centre study. BMC Endocr Disord 2017; 17: 39


 PDF
PDF  Views
Views  Share
Share

