Combined Modality Treatment with “Dexamethasone, Methotrexate, Ifosfamide, L-Asparaginase, and Etoposide ” Chemotherapy and Involved Field Radiotherapy for Early Stage Natural Killer/T Cell Lymphoma with Local Tumor Invasiveness: A Single-institution Study from India
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 67-72
DOI: DOI: 10.4103/ijmpo.ijmpo_60_17
Abstract
Context:?Patients with early stage extranodal natural killer/T-cell lymphoma, nasal type (ES-NKTCL) and local tumor invasiveness (LTI) show poor treatment outcomes with standard approaches. Dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) is an intensive, highly active protocol mainly studied in advanced/recurrent disease. No prior study has utilized this protocol in high-risk ES-NKTCL.?Methods:?Between 2011 and 2016, all patients with ES-NKTCL with LTI at presentation were uniformly treated at our institute with a combination of SMILE chemotherapy for 5?6 cycles, and involved-field radiotherapy (IFRT). Records of these patients were retrospectively reviewed.?Results:?Sixteen patients were identified, 69% stage IE and 31% stage IIE. The majority of patients had B-symptoms (75%), paranasal sinus (PNS) invasion (81%), facial skin invasion (56%), palatal perforation (69%), or orbital extension (56%). 12/16 had B-symptoms, and 6/16 had elevated lactate dehydrogenase. All patients received the entire planned 5?6 cycles. IFRT was delivered after a mean 4 cycles. Complete remission was achieved in 13/15 (87%) patients. At a median follow up of 18.5 months, 1-year progression-free survival and overall survival was 84% and 94%, respectively. Grade 3?4 toxicity was seen in 81%, most commonly neutropenia (75%), anemia (44%), and thromobocytopenia (31%). Six patients required dose adjustments (predominantly in the first 1 or 2 cycles). No treatment-related mortality was noted.?Conclusion:?SMILE with RT is a toxic but tolerable protocol for ES-NKTCL with LTI with high efficacy. Prospective studies are warranted.
Keywords
Asparaginase - chemotherapy - lymphoma - methotrexate - natural killer cells
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:?Patients with early stage extranodal natural killer/T-cell lymphoma, nasal type (ES-NKTCL) and local tumor invasiveness (LTI) show poor treatment outcomes with standard approaches. Dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) is an intensive, highly active protocol mainly studied in advanced/recurrent disease. No prior study has utilized this protocol in high-risk ES-NKTCL.?Methods:?Between 2011 and 2016, all patients with ES-NKTCL with LTI at presentation were uniformly treated at our institute with a combination of SMILE chemotherapy for 5?6 cycles, and involved-field radiotherapy (IFRT). Records of these patients were retrospectively reviewed.?Results:?Sixteen patients were identified, 69% stage IE and 31% stage IIE. The majority of patients had B-symptoms (75%), paranasal sinus (PNS) invasion (81%), facial skin invasion (56%), palatal perforation (69%), or orbital extension (56%). 12/16 had B-symptoms, and 6/16 had elevated lactate dehydrogenase. All patients received the entire planned 5?6 cycles. IFRT was delivered after a mean 4 cycles. Complete remission was achieved in 13/15 (87%) patients. At a median follow up of 18.5 months, 1-year progression-free survival and overall survival was 84% and 94%, respectively. Grade 3?4 toxicity was seen in 81%, most commonly neutropenia (75%), anemia (44%), and thromobocytopenia (31%). Six patients required dose adjustments (predominantly in the first 1 or 2 cycles). No treatment-related mortality was noted.?Conclusion:?SMILE with RT is a toxic but tolerable protocol for ES-NKTCL with LTI with high efficacy. Prospective studies are warranted.
Keywords
Asparaginase - chemotherapy - lymphoma - methotrexate - natural killer cells
Introduction
Extranodal natural killer/T-cell lymphoma, nasal type (NKTCL) is an aggressive lymphoma with a strong geographical predilection seen predominantly in Asian and South American populations. These lymphomas generally present as destructive lesions of the nose and nasopharynx and surrounding areas.[1] The majority of patients (70%?90%) present with localized disease restricted to this location (Ann Arbor stage IE/IIE) and are considered to have early stage NKTCL (ES-NKTCL). The optimal therapy of these patients is controversial. Radiotherapy (RT) is generally considered mandatory for cure, with chemotherapy added either sequentially or concurrently.[2] Very low-risk patients may do well with RT alone.[3]
Among the most important prognostic factors in ES-NKTCL is local tumor invasiveness (LTI). This entity is generally defined as tumor that extends to adjacent structures such as PNS, skin, or palate. Multiple studies indicate that LTI is among the most consistent and powerful prognostic factors in ES-NKTCL.[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15] The significantly worse treatment outcome of ES-NKTCL with LTI has led some oncologists to propose that the staging of ES-NKTCL be modified to reflect the importance of this prognostic factor,[16] and treatment be tailored according to risk scores that take LTI into account.[3],[5]
NKTCL patients respond poorly to anthracycline-based chemotherapy due to high expression of P-glycoprotein in the malignant cells resulting in a multi-drug resistant (MDR) phenotype.[2],[17] L-asparaginase based protocols have been attempted in ES-NKTCL with good results. However, the number of patients with LTI have been few or unreported in these studies.[18],[19] In view of the poor outcomes of patients with LTI, they are candidates for consideration of more intensive treatment approaches, as are generally used in advanced disease. The protocol dexamethasone, high-dose methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) is among the most aggressive non-MDR regimens for ES-NKTCL. The protocol has been used predominantly in the advanced/recurrent setting, and experience in ES-NKTCL is limited.[20],[21],[22],[23] We hypothesized that use of SMILE (a more aggressive protocol) as a component of multimodal therapy may lead to improved clinical outcomes in this group of high-risk patients.
Materials and Methods
Patient selection and staging evaluation
Treatment records of all patients with histopathologically proven ES-NKTCL, who were treated at our cancer center from 2011 to 2016 were retrospectively reviewed. All patients underwent a complete baseline staging including complete blood count, liver and kidney function tests, serum lactate dehydrogenase (LDH), contrast-enhanced computed tomography (CECT) of PNS, neck, chest, abdomen and pelvis; nasal endoscopy with biopsy; and bone marrow aspiration and biopsy. Additional evaluation including magnetic resonance imaging or whole body 18-F fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET-CT) was done as felt necessary. LTI was defined as the presence of disease extending into neighboring structures or organs, or involvement of multiple, contiguous primary sites. International Prognostic Index (IPI) and Korean Prognostic Index (KPI) was calculated for all patients.[14]
Treatment
All patients with ES-NKTCL with LTI at presentation were uniformly treated at our institute with a combination of chemotherapy with SMILE regimen [Table 1] for 5?6 cycles, and involved-field radiotherapy (IFRT). RT (45?50 gray/25 fractions/5 weeks) was introduced in the treatment protocol usually after the completion of 3?4 cycles of initial chemotherapy. Patients required in-patient admission for each cycle of chemotherapy, whereas intramuscular L-asparaginase and subcutaneous growth factors were given on out-patient basis as per protocol. Patients with poor performance status at presentation (PS 3?4) or those who suffered significant toxicity underwent dose modifications of offending chemotherapeutic drugs. All patients received prophylactic antimicrobial prophylaxis with acyclovir and cotrimoxazole (withheld during methotrexate administration and introduced after clearance) during the entire duration of therapy. Local RT was usually planned by three-dimensional conformal technique. Intensity modulated RT was used when necessary for sparing of critical organs at risk. The response was assessed by CECT scan; patients with clinical suspicion of an active disease or equivocal findings on CECT underwent FDG PET-CT scan and histological examination, if feasible. Toxicity was assessed as per Common Terminology Criteria for Adverse Events version 4.0.
|
Drug |
Daily dose |
Route |
Days of protocol |
|---|---|---|---|
|
G-CSF ? Granulocyte colony stimulating factor; HDMTX ? High dose methotrexate; IM ? Intramuscular; IV ? Intravenous; SC ? Subcutaneous; WBC ? White blood cell |
|||
|
HDMTX |
2 g/m2 |
IV over 6 h |
1 |
|
Leucovorin |
15 mg x 4 |
IV or oral |
2-4 |
|
Ifosfamide |
1.5 g/m2 |
IV |
2-4 |
|
Mesna |
300 mg/m2 x 3 |
IV |
2-4 |
|
Dexamethasone |
40 mg |
IV or oral |
2-4 |
|
Etoposide |
100 mg/m2 |
IV |
2-4 |
|
L-asparaginase (Escherichia coli) |
6000 IU/m2 |
IM |
8, 10, 12, 14, 16, 18, 20 |
|
G-CSF |
300 mcg |
SC |
6 till WBC >5000 |
|
Parameter |
n (%) |
|---|---|
|
B-symptoms -?Defined as unexplained weight loss of >10% of the body weight in the 6 months previous to presentation; unexplained fever with temperature >38?C; or night sweats. ECOG ? Eastern Cooperative Oncology Group; IPI ? International Prognostic Index; KPI ? Korean Prognostic Index; LDH ? Lactate dehydrogenase |
|
|
Age in years (median, range) |
31(19-55) |
|
Female gender |
6 (38) |
|
Symptom duration in months (median, range) |
5.5 (1-24) |
|
B-symptoms |
12 (75) |
|
Stage |
|
|
IE |
11 (69) |
|
IIE |
5 (31) |
|
IPI |
|
|
0 |
9 (56) |
|
1 |
3 (19) |
|
2 |
4 (25) |
|
3 |
0 |
|
4 |
0 |
|
5 |
0 |
|
KPI |
|
|
0 |
3 (19) |
|
1 |
6 (38) |
|
2 |
5 (31) |
|
3 |
2 (13) |
|
4 |
0 |
|
ECOG performance status |
Available for |
|
13/16 patients |
|
|
0 |
0 |
|
1 |
7 (54) |
|
2 |
4 (31) |
|
3 |
2 (15) |
|
4 |
0 |
|
Palatal perforation |
11 (69) |
|
Paranasal sinus invasion |
13(81) |
|
Orbital extension |
9 (56) |
|
Facial skin invasion |
9 (56) |
|
Oropharyngeal extension |
3 (19) |
|
Intracranial extension |
2 (13) |
|
Lymph node involvement |
5 (31) |
|
Elevated LDH |
6 (38) |
|
Grade 3-4 toxicity |
n (%) |
|---|---|
|
Any |
13 (81) |
|
Neutropenia |
12 (75) |
|
Febrile neutropenia |
4 (25) |
|
Thrombocytopenia |
5 (31) |
|
Anemia |
7 (44) |
|
Mucositis |
1 (6) |
|
Encephalopathy |
1 (6) |
|
Anaphylaxis |
1 (6) |
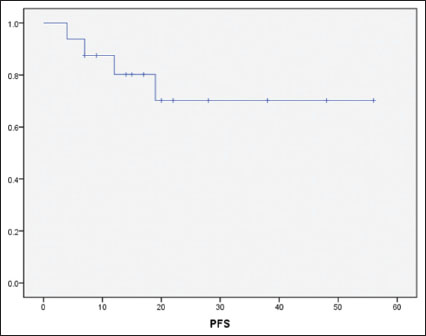
|?Figure.1Progression-free survival for the cohort by Kaplan-Meier analysis
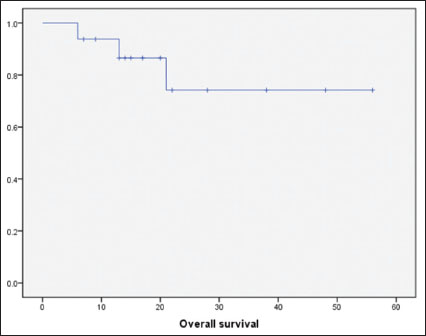
|?Figure.2Overall survival for the cohort by Kaplan-Meier analysis
Discussion
ES-NKTCL with LTI is an aggressive, difficult to treat malignancy. Our cancer center, located in North India, provides-free service to socioeconomically vulnerable populations from neighboring states. Due to limited cancer awareness, the paucity of diagnostic and treatment facilities and economic limitations, the vast majority of patients with NKTCL attending our center present late with either ES-NKTCL with LTI or with advanced-stage NKTCL. The heavy disease burden is reflected in the fact that the majority had features such as PNS invasion (81%), facial skin invasion (56%), palatal perforation (69%), or orbital extension (56%).
The treatment of these patients is challenging. The majority of previous studies of ES-NKTCL which have looked at LTI as a prognostic factor have reported poorer outcomes for these patients.[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15] In fact, it has been commented that the prognosis of these patients is closer to advanced stage NKTCL than early stage.[16] The natural conclusion that follows is that standard treatment approaches for these patients may be inadequate and more aggressive and intensive therapy should be attempted. This leads to the question of how this intensification may be accomplished. Anthracycline-based chemotherapy is now considered less effective and modern chemotherapy for patients with NKTCL includes a backbone of L-asparaginase and other non-MDR drugs.[2] Ways to combine non-MDR chemotherapy with RT include concurrent chemoradiation (CCRT) or sequential chemotherapy and RT. CCRT with non-MDR protocols has delivered improved outcomes (78% OS at 2 years with dexamethasone, etoposide, ifosfamide, and carboplatin - ?DeVIC?; 86% OS at 3 years with etoposide, ifosfamide, cisplatin, and dexamethasone - ?VIPD?) in unselected ES-NKTCL patients compared to historical controls.[24],[25] Timely CCRT is often difficult to implement outside a trial setting due to logistic reasons; further, tolerability in patients with the active up front disease is also a concern. Thus, sequential therapy is generally preferred.[2] Sequential therapy with asparaginase, vincristine, and prednisolone or GELOX with RT have resulted in 2-year OS rates of 86%?89%.[18],[19] However, in all these studies, patients with LTI have been very few [19] or unreported.[18],[24],[25] The poor prognosis and aggressive course of ES-NKTCL with LTI indicate that these patients warrant a more intensive approach, analogous to that utilized for advanced stage NKTCL. Hence, we selected SMILE, which is considered the most aggressive chemotherapy protocol for NKTCL to treat this population.
By utilizing a combination of SMILE and IFRT, we achieved a 1-year PFS and OS rates of 84% and 94%, respectively. The 2-year PFS and OS rates were 70% and 74%, respectively. These outcomes are notably better than described in historical series of ES-NKTCL with LTI.[6],[7],[8],[9],[10],[12] Longer follow up will clarify whether this favorable clinical outcome will be maintained, but it is notable that the majority of relapses of NKTCL tend to occur within the first 2 years.[26]
An important limitation of SMILE is the high toxicity associated with this protocol.[20],[21],[22] In our study, 81% of patients suffered grade 3?4 toxicity, predominantly hematological. However, the majority of patients could complete the planned chemotherapy, and there was no treatment-related mortality. While it is undebatable that SMILE is a toxic protocol, we believe that if managed carefully in large volume centers with considerable experience and good quality supportive care, toxicity is manageable [Table 4].[27] For instance, Yamaguchi?et al. noted that modification of their infection surveillance protocol was useful in preventing deaths among patients on SMILE regimen.[20] To sum up, adequate experience, appropriate use of chemotherapeutic agents, dose modifications whenever needed, prophylactic antimicrobial therapy and close surveillance for infections can help in achieving a good clinical outcome in patients with ES-NKTCL with this aggressive combined modality treatment.[23],[27]
|
Reference |
Curren study |
|||
|---|---|---|---|---|
|
[20] |
[22] |
[21] |
||
|
NR ? Not reported; SMILE ? Dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide |
||||
|
Number of patients |
38 |
87 |
21 |
16 |
|
Any Grade III-IV |
100% |
NR |
NR |
81% |
|
Treatment-related deaths |
5% |
6% |
At least 14% |
0% |
|
Neutropenia |
100% |
67% |
86% |
75% |
|
Febrile neutropenia/infection |
61% |
31% |
NR |
25% |
|
Thrombocytopenia |
64% |
42% |
52% |
31% |
|
Anemia |
50% |
NR |
29% |
44% |
|
Encephalopathy |
3% |
NR |
NR |
6% |
|
Mucositis |
13% |
NR |
10% |
6% |
|
Anaphylaxis |
NR |
1% |
NR |
6% |
|
Nephrotoxicity |
5% |
1% |
5% |
0% |
|
Hyperbilirubinemia |
11% |
7% |
10% |
0% |
|
Comments |
No deaths after modification of infection surveillance protocol and inclusion criteria |
4/5 patients who died had refractory lymphoma |
Unusually high toxicity in SMILE arm[27] 19% did not complete a single cycle, 14% died during cycle 1, only 57% received >4 cycles |
|

|?Figure.1Progression-free survival for the cohort by Kaplan-Meier analysis

|?Figure.2Overall survival for the cohort by Kaplan-Meier analysis
References
- iang X, Graham DK.?Natural killer cell neoplasms. Cancer 2008; 112: 1425-36
- se E, Kwong YL.?How I treat NK/T-cell lymphomas. Blood 2013; 121: 4997-5005
- ang Y, Zhu Y, Cao JZ, Zhang YJ, Xu LM, Yuan ZY.?et al.?Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: Analysis from a multicenter study. Blood 2015; 126: 1424-32
- i XW, Zhang WW, Li ZM, Huang JJ, Xia Y, Sun P.?et al.?The extent of local tumor invasion predicts prognosis in stage IE nasal natural killer/T-cell lymphoma: A novel T staging system for risk stratification. Ann Hematol 2015; 94: 1515-24
- ang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY, Xu LM.?et al.?Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia 2015; 29: 1571-7
- ang H, Jin J, Wang WH, Wang SL, Zhou LQ, Li YX.?Prognostic factors and treatment outcomes for patients with stage II extranodal nasal-type natural killer/T-cell lymphoma of the upper aerodigestive tract. Leuk Lymphoma 2014; 55: 1832-7
- im JY, Lee SW, Lee JH, Suh C, Yoon DH, Lee BJ.?et al.?Stage IE/IIE extranodal NK/T-cell lymphoma arising in the nasal cavity: Analysis of CT findings and their prognostic value. Clin Radiol 2013; 68: e384-90
- a II, Kang HJ, Park YH, Lee SS, Yoo HJ, Choe DH.?et al.?Prognostic factors for classifying extranodal NK/T cell lymphoma, nasal type, as lymphoid neoplasia. Eur J Haematol 2007; 79: 1-7
- i YX, Yao B, Jin J, Wang WH, Liu YP, Song YW.?et al.?Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 2006; 24: 181-9
- Kim TM, Park YH, Lee SY, Kim JH, Kim DW, Im SA.?et al.?Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood 2005; 106: 3785-90
- You JY, Chi KH, Yang MH, Chen CC, Ho CH, Chau WK.?et al.?Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: A single institute survey in Taiwan. Ann Oncol 2004; 15: 618-25
- Li YX, Coucke PA, Li JY, Gu DZ, Liu XF, Zhou LQ.?et al.?Primary non-Hodgkin's lymphoma of the nasal cavity: Prognostic significance of paranasal extension and the role of radiotherapy and chemotherapy. Cancer 1998; 83: 449-56
- d">13?Robbins KT, Fuller LM, Vlasak M, Osborne B, Jing BS, Velasquez WS.?et al.?Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer 1985; 56: 814-9
- Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH.?et al.?Extranodal natural killer T-cell lymphoma, nasal-type: A prognostic model from a retrospective multicenter study. J Clin Oncol 2006; 24: 612-8
- d">15?Logsdon MD, Ha CS, Kavadi VS, Cabanillas F, Hess MA, Cox JD.?Lymphoma of the nasal cavity and paranasal sinuses: Improved outcome and altered prognostic factors with combined modality therapy. Cancer 1997; 80: 477-88
- Kim TM, Heo DS.?Extranodal NK/T-cell lymphoma, nasal type: New staging system and treatment strategies. Cancer Sci 2009; 100: 2242-8
- d">17?Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T.?et al.?Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 1995; 76: 2351-6
- Wang L, Wang ZH, Chen XQ, Li YJ, Wang KF, Xia YF.?et al.?First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013; 119: 348-55
- Jiang M, Zhang H, Jiang Y, Yang Q, Xie L, Liu W.?et al.?Phase 2 trial of ?sandwich? L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer 2012; 118: 3294-301
- d">20?Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C.?et al.?Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin Oncol 2011; 29: 4410-6
- Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X.?et al.?DDGP versus SMILE in newly diagnosed advanced natural Killer/T-Cell lymphoma: A randomized controlled, multicenter, open-label study in China. Clin Cancer Res 2016; 22: 5223-8
- Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E.?et al.?SMILE for natural killer/T-cell lymphoma: Analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012; 120: 2973-80
- Chan A, Tang T, Ng T, Shih V, Tay K, Tao M.?et al.?To SMILE or not: Supportive care matters. J Clin Oncol 2012; 30: 1015-6
- d">24?Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y.?et al.?Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009; 27: 5594-600
- Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J.?et al.?Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol 2009; 27: 6027-32
- Kwong YL.?Natural killer-cell malignancies: Diagnosis and treatment. Leukemia 2005; 19: 2186-94
- Gupta VG, Gogia A.?DDGP versus SMILE in NK/T-Cell Lymphoma-Letter. Clin Cancer Res 2016; 22: 4271


 PDF
PDF  Views
Views  Share
Share

