Comparative evaluation of various cytomorphological grading systems in breast carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 79-84
DOI: DOI: 10.4103/0971-5851.180141
Abstract
Background: The diagnosis of breast carcinoma can be reliably made by fine needle aspiration cytology (FNAC). Grading usually done in histological samples for the selection of therapy but not in cytology. Various cytological grading systems have been proposed; however, none of them is presently considered the gold standard to predict the prognosis. Aim: This study was undertaken to evaluate various 3-tier cytological grading systems and to determine the best possible system corresponds to the histological grading proposed by Elston and Ellis based on the method by Nottingham modification of Scarff-Bloom-Richardson (SBR) method. Materials and Methods: In this retrospective study, 94 cases of breast carcinoma FNACs were graded using six cytological grading systems and compared with SBR method. Concordance, association, and correlation studies were done to select best possible cytological grading system. The interobserver reproducibility among the six grading systems was also assessed. Results: Robinson method showed best correlation (r = 0.801; P = 0.0001 and t = 0.783; P = 0.0001), maximum percent agreement (83/94 cases; 88.3%), and a substantial kappa value of agreement (k = 0.737) with the Nottingham modification of SBR grading system followed by Mouriguand method. Taniguchi system showed better interobserver agreement (87.2%; k= 0.738). Conclusions: This study showed that all six cytological grading systems correlated positively with SBR method. However, Robinson's grading system demonstrated the best concordance, correlation, and substantial Kappa value of the agreement with the histological grading by SBR method in comparison to other 3-tier cytological grading systems. Hence, in conclusion, this grading should be routinely incorporated in the cytology reports as it correlates well with histological grade. Despite various cytological grading systems, Robinson's method is simple, more objective, and reproducible, hence being preferable for routine use.
Keywords
Breast carcinoma - cytological grading - Nottingham modification of Scarff-Bloom-Richardson method - Robinson's gradingPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The diagnosis of breast carcinoma can be reliably made by fine needle aspiration cytology (FNAC). Grading usually done in histological samples for the selection of therapy but not in cytology. Various cytological grading systems have been proposed; however, none of them is presently considered the gold standard to predict the prognosis.
Aim:
This study was undertaken to evaluate various 3-tier cytological grading systems and to determine the best possible system corresponds to the histological grading proposed by Elston and Ellis based on the method by Nottingham modification of Scarff-Bloom-Richardson (SBR) method.
Materials and Methods:
In this retrospective study, 94 cases of breast carcinoma FNACs were graded using six cytological grading systems and compared with SBR method. Concordance, association, and correlation studies were done to select best possible cytological grading system. The interobserver reproducibility among the six grading systems was also assessed.
Results:
Robinson method showed best correlation (ρ = 0.801; P = 0.0001 and τ = 0.783; P = 0.0001), maximum percent agreement (83/94 cases; 88.3%), and a substantial kappa value of agreement (κ = 0.737) with the Nottingham modification of SBR grading system followed by Mouriguand method. Taniguchi system showed better interobserver agreement (87.2%; κ = 0.738).
Conclusions:
This study showed that all six cytological grading systems correlated positively with SBR method. However, Robinson's grading system demonstrated the best concordance, correlation, and substantial Kappa value of the agreement with the histological grading by SBR method in comparison to other 3-tier cytological grading systems. Hence, in conclusion, this grading should be routinely incorporated in the cytology reports as it correlates well with histological grade. Despite various cytological grading systems, Robinson's method is simple, more objective, and reproducible, hence being preferable for routine use.
INTRODUCTION
In women, breast carcinoma is one of the most common cancers in the world and is second most common malignancy in India.[1,2] The histological grading proposed by Elston and Ellis using Nottingham modification of Scarff-Bloom-Richardson (SBR) method for breast carcinoma is widely accepted tumor grading system, and it is a useful, sensitive guide for selecting neoadjuvant therapy and has been found to have a good prognostic correlation.[3] However, cytological grading of breast carcinoma is sparingly used and reported. Selection of neoadjuvant therapy and assessment of the tumors without surgery can be achieved by incorporation of cytological grading system in fine needle aspiration cytology (FNAC) smears of breast carcinoma and thereby morbidity due to surgical intervention, especially in low-grade tumors can be avoided.[4,5] Previously, the role of FNAC has been challenged by results obtained with core needle biopsy (CNB) that seems more robust than FNAC. In general, CNB is now preferred in the first line of diagnosis.[6] However, CNB carries complications such as pain (1.7-3.7%), vasovagal reactions (1%), severe bleeding (0.72%), infections (0.15%), and hematoma (0.09%).[7] FNAC has more advantages than CNB such as minimal invasiveness and minimal discomfort that could be interesting for aged or frailty patients with comorbidities.[8] FNAC is also easier/safer in certain lesions such as very small lesions, lesions just under the skin or very close to the chest wall compared to CNB. In addition, FNAC maintains tactile sensitivity, allows multidirectional passes allowing a broader sampling of lesion and immediate reporting where necessary.[9] Use of FNAC is essentially true in underdeveloped/developing countries, where the tissue CNB still is not used as a standard practice to sample newly diagnosed cases of carcinoma breast.[10] The National Cancer Institute, Bethesda, sponsored conference had also recommended that in FNAC reports of breast carcinoma, tumor grade should be incorporated for prognostication.[11] It was also emphasized that the cytological grading system on FNAC smears should correspond to the histological grading system.
There are various cytological grading systems, namely, Robinson's et al.,[12] Mouriquand's and Pasquier[13,14] Taniguchi et al.,[4] Fisher's modification of Black's nuclear grading scheme,[15] Khan et al.,[16] and Howell et al.[17] grading systems can be applied on FNAC smears of breast carcinoma. Some authors have also compared and correlated the outcome of these grading methods with the biological behavior, similar to SBR method. However, none of the methods is considered the gold standard for the cytological grading and also there is no agreement among pathologists and clinicians to accept one of them as effective as SBR grading system.[18] In this study, we evaluated six 3-tier cytological grading systems and correlated with SBR method to determine best cytological grading scheme correspond to the histologic grading system.
MATERIALS AND METHODS
After obtaining approval from institutional ethical committee, a total of 94 cases of breast carcinoma diagnosed by FNAC from July 2012 to January 2015 were included, and histopathological correlation was done in this retrospective study.
FNAC smears were stained by May-Grunwald Giemsa and hematoxylin and eosin (H and E) were studied and graded independently by two pathologists using six 3-tier grading systems, namely, Robinson's et al. grading,[12] Mouriquand's and Pasquier grading,[13,14] Taniguchi et al. grading,[4] Fisher's modification of Black's nuclear grading,[15] Khan et al. grading,[16] and Howell et al. grading.[17]
In Robinson's et al. grading system,[12] six different cytological parameters such as cell dissociation, cell size, uniformity, nucleoli, nuclear margin, and chromatin were given a score of 1-3 and smears that scored in the range of 6-11 were Graded I, smears with a score of 12-14 were Graded II, and smears with a score of 15-18 were Graded III [Figure 1].
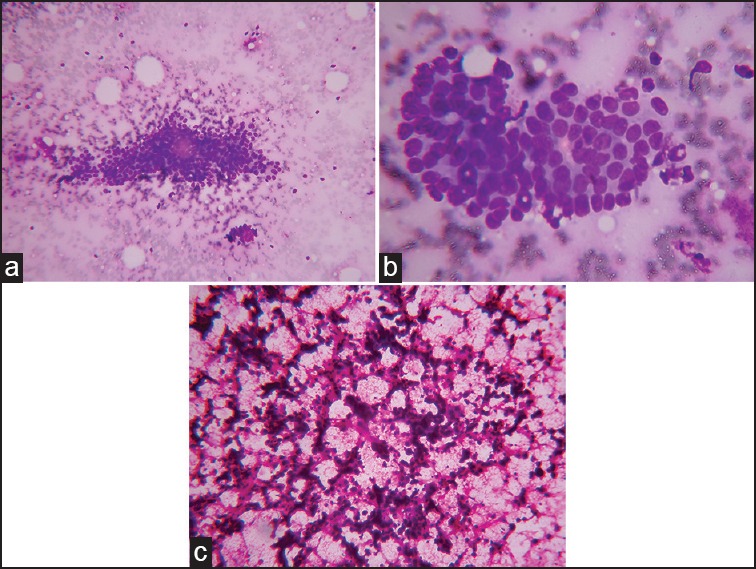
| Fig. 1 Robinson's grade. (a) Robinson's Grade I showing monomorphic cell cluster with vesicular nuclei (May-Grunwald Giemsa, ×100). (b) Robinson's Grade II showing mild to moderate pleomorphic tumor cells (May-Grunwald Giemsa, ×400). (c) Robinson's Grade III showing singly scattered cells with marked pleomorphism (H and E, ×100)
Mouriquand's and Pasquier grading,[13,14] gave a score of 0-3 to cellular and nuclear features, chromatin, and mitosis. The combined score < 5 were considered as Grade I, a score 6-9 were considered as Grade II, and a score >10 were considered as Grade III [Figure 2a].
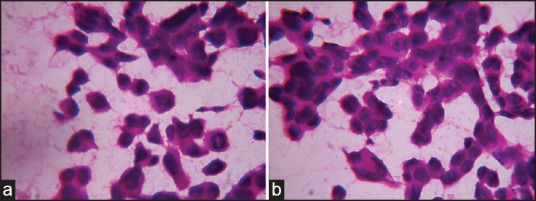
| Fig. 2 (a) Mouriquand's Grade III showing large cells with hyperchromatic nuclei and mitosis (H and E, ×400). (b) Fisher's modification of Black's nuclear Grading III showing enlarged tumor cells with prominent nucleoli (H and E, ×400)\
Taniguchi et al. grading[4] included seven cytological parameters such as necrosis, cellular size, nuclear-cytoplasmic ratio, nuclear pleomorphism, nucleoli, chromatin granularity, and density of chromatin. All the parameters were scored from 1 to 3 except necrosis which was scored 0 or 1 and total in the range of 6-9 were Grade I, 10-11 were Grade II, and 12-19 were Grade III.
In Fisher's modification of Black's nuclear grading,[15] five parameters such as nuclear shape, chromatin, nucleoli, mitosis, and nuclear size were Graded I-III [Figure 2b].
In Khan et al. grading,[16] six parameters such as pleomorphism, nuclear size, nuclear margins, nucleoli, naked tumor nuclei, and mitotic count were given a score of 1-3, and the tumors were Graded I if the combined score was 6-10, II for a score ranging from 11 to 14, and III for score from 15 to 18.
Howell et al. grading system[17] is similar to the SBR method with modification to the mitotic count as score 1 for 0-1/10 high power fields (HPFs), 2 for 2-4/10 HPF, and 3 for > 5/10 HPF. The Grades were given as I, II, and III for scores in the range of 3-5, 6-7, and 8-9, respectively.
Histopathological grading was done on the postoperative mastectomy specimens using the Nottingham modification of SBR method[3] in H and E stained sections [Figure 3]. Mitotic figures were counted and scored using an Olympus CH20i microscope with HPF diameter 0.45 mm.
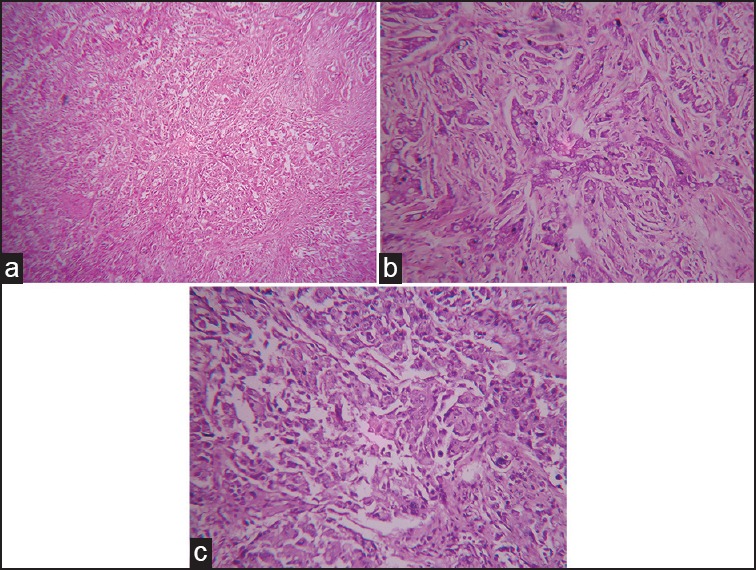
| Fig. 3 Nottingham modification of Scarff-Bloom-Richardson grading. (a) Grade I invasive ductal carcinoma, not otherwise specified (H and E, ×100). (b) Grade II invasive ductal carcinoma, not otherwise specified (H and E, ×400). (c) Grade III invasive ductal carcinoma, not otherwise specified (H and E, ×400)
Statistical analysis
The results were tabulated, and statistical analyses were done with the IBM SPSS Statistics for Windows (version 20.0. Armonk, New York: IBM Corporation). Association between different grading systems was assessed by Chi-square test. Correlation of various cytological grading system of FNAC smears were done by Spearman's correlation coefficient (r) and Kendall's tau-b rank correlation coefficient (t). Agreement or concordance was assessed by kappa measurement of agreement (k). The P value of 0.05 or less was considered for statistical significance.
RESULTS
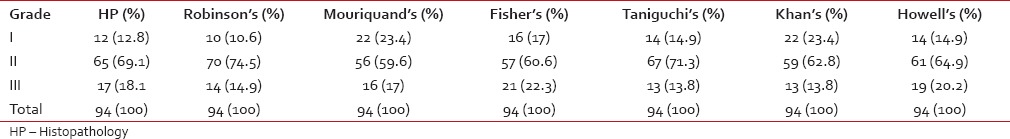
Ninety-four cases of invasive ductal carcinoma, not otherwise specified were studied. Overall, the majority of cases were Grade II followed by Grade I and III. The distribution of cases according to various 3-tier cytological grading systems and histological grading is shown in Table 1.
Table 1
Distribution of cases according to various 3-tier cytological grading and histological grading (n = 94)
 The association of each of the cytological grading systems and the histological grading by SBR method was found to be highly significant with a P < 0.0001 as measured by Chi-square test. Spearman rank correlation coefficient (r) and Kendall's tau-b rank correlation coefficient (t) revealed strong and positive correlation of all cytological grading systems with histological grading is shown in Table 2. Robinson's grading system showed the highest concordance (88.3%, 83/94 cases), and agreement (k value 0.737, substantial agreement) with the histological grading.
The association of each of the cytological grading systems and the histological grading by SBR method was found to be highly significant with a P < 0.0001 as measured by Chi-square test. Spearman rank correlation coefficient (r) and Kendall's tau-b rank correlation coefficient (t) revealed strong and positive correlation of all cytological grading systems with histological grading is shown in Table 2. Robinson's grading system showed the highest concordance (88.3%, 83/94 cases), and agreement (k value 0.737, substantial agreement) with the histological grading.Table 2
Correlation and concordance analysis between the cytological grading system and the histological grading
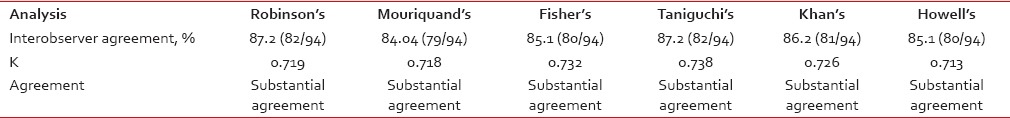
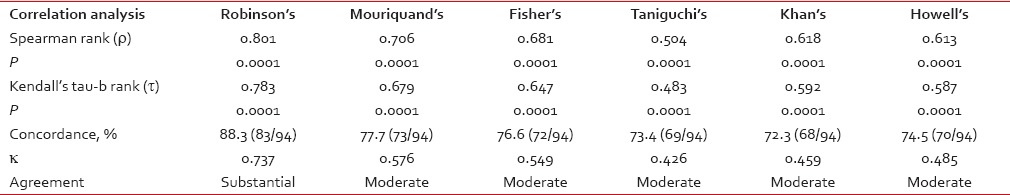 The interobserver agreement was analyzed by Kappa (k) measurement of agreement, and the result is shown in Table 3. The percent of agreement was maximum in both Robinson's grading and Taniguchi's grading and least in Mouriquand's grading. There was only scanty variation in the k value, all the 3-tier cytological grading system showed substantial agreement between two pathologists.
The interobserver agreement was analyzed by Kappa (k) measurement of agreement, and the result is shown in Table 3. The percent of agreement was maximum in both Robinson's grading and Taniguchi's grading and least in Mouriquand's grading. There was only scanty variation in the k value, all the 3-tier cytological grading system showed substantial agreement between two pathologists.Table 3
Analysis of interobserver agreement for various 3-tier cytological grading systems
DISCUSSION
In both nonneoplastic and neoplastic breast lesions, FNAC generally considered as a rapid, reliable, and safe diagnostic tool. FNAC is the initial method of pathological assessment as a component of the triple test in the diagnosis of palpable breast lesions in developing countries.
A Recent review showed that FNAC of the breast has a sensitivity ranging from 76% to 99% and specificity ranging from 60% to 100%.[19] Cytological grading of breast carcinoma not only provides a diagnosis but also information about prognosis without additional morbidity or expense of core or excision biopsy to the patients, especially in resource-limited situations.[10] A cytological evaluation of the prognostic markers is important, and it is useful in patients with inoperable tumors and in high-risk surgery.[20] There are many cytological grading systems have been proposed by various authors, but none have been implemented in cytology reports. Many authors in their studies have compared the well-known Robinson's grading system with SBR method; however, only a few studies have compared other 3-tier cytological grading systems with histologic grading. In this study, we evaluated six 3-tier cytological grading systems including Robinson's grading.
In a study done by Das et al.,[21] on comparison of histologic grading with Robinson's and Mouriquand's grading system, both method observed 71.2% concordance, but they considered Robinson's grading method as a better choice due to its simplicity, specificity, and better reproducibility. The concordance rate of Robinson's grading in this study was 88.3%. It was almost similar in most of the published studies; 57% by Robinson et al.,[12] 71.2% by Das et al.,[21] 65% by Chhabra et al.,[22] 83% by Meena et al.,[23] 88.89% by Bhargava et al.,[5] 81% by Sinha et al.,[24] 88% by Khan et al.,[25] 64% by Lingegowda et al.,[26] 77.19% by Saha et al.,[18] and 77.7% by Einstien et al.[27]
The concordance rate for Mouriquand's grading in this study was 77.7%. It was similar to studies done by Saha et al.[18] and Einstien et al.[27] (77.19% and 68%, respectively).
Different studies in the past have observed different agreement on a comparison of Fisher's modification of Black' nuclear grading with histological grading by SBR method. It was 76.3% by Einstien et al.,[27] 70.18% by Saha et al.,[18] 95% by Dabbs,[28] 70.37% by Zoppi et al.,[29] 77.78% by Bhargava et al.,[5] and 76.6% in this study which were almost similar to above mentioned studies.
Taniguchi's grading showed 73.4% concordance in our study, whereas Taniguchi et al.[4] observed 44.4%, Saha et al.[18] observed 75.44%, and Einstien et al.[27] observed 66.6%.
Our study showed a concordance rate of 72.3% for Khan's grading, whereas in Khan et al.,[16] Saha et al.,[18] and Einstien et al.[27] studies, concordance rate were 97.14%, 66.67%, and 72.2%, respectively.
We observed 74.5% concordance rate for Howell's grading which was a modification of the Nottingham's SBR grading; however, it was 57.1% by Howell et al.,[17] 50% by Bhargava et al.,[5] 82% by Lingegowda et al.,[26] 63.16% by Saha et al.,[18] and 69.4% by Einstien et al.[27]
In the studies done by Frias et al.[30] and Bhargava et al.[5] showed a statistically significant association between Robinson's grading and SBR histological grading (P < 0.0005 and P < 0.001 respectively) similar to this study (P < 0.0001).
Correlation of cytological grading by Robinson's system with histological grading showed a correlation coefficient of 0.537 by Chhabra et al.,[22] 0.774 by Frias et al.,[30] 0.519 by Lingegowda et al.,[26] 0.799 by Saha et al.[18] and 0.738 by Einstien et al.[27] indicating strong positive correlation as observed in our study.
Only scanty studies are available in the literature for the correlation of all the 3-tier cytological grading systems with histological grading and also for the interobserver agreement. Our study showed that all the six 3-tier cytological grading systems strongly and positively correlated with histological grading. The interobserver agreement of 74.3% for histological grading and 65.7% for cytological grading was found in Howell et al. study.[17] Lingegowda et al.[26] found 98% interobserver agreement for Robinson's system compared to 92% for Howell's system. In our study, interobserver agreement for the all cytological grading system showed substantial agreement, k value ranging from 0.713 to 0.738 similar to Saha et al.[18] and Einstien et al.[27] Comparison of correlation coefficient analysis, concordance, and interobserver agreement of this study with other studies is shown in Table 4.
Table 4
Comparison of correlation, concordance and interobserver agreement with other studies
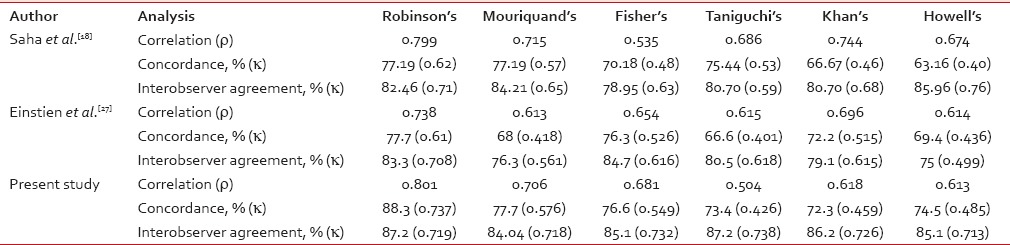 In our study, Robinson's grading system showed best concordance rate of 88.3% (83/94 cases), k value of agreement 0.737 with substantial range, and the best correlation of ρ = 0.801; P = 0.0001 and τ=0.783; P = 0.0001 with histological grading. It also showed good interobserver agreement with τ value of 0.719 (87.2%, 82/94 cases).
In our study, Robinson's grading system showed best concordance rate of 88.3% (83/94 cases), k value of agreement 0.737 with substantial range, and the best correlation of ρ = 0.801; P = 0.0001 and τ=0.783; P = 0.0001 with histological grading. It also showed good interobserver agreement with τ value of 0.719 (87.2%, 82/94 cases).Even though FNAC considered as rapid, relatively inexpensive, and less traumatic procedure, it has certain limitations includes inadequate cell yield which mainly occur due to sclerotic fibroadenomas, sclerosing ductal carcinoma, and infiltrating lobular carcinoma, less reliable at differentiating invasive cancer from ductal carcinoma in situ and moreover, it require considerable experience for the interpretation of smears.
CONCLUSIONS
This study showed that all six cytological grading systems correlated positively with SBR method. However, Robinson's grading system demonstrated the best concordance, correlation, and substantial Kappa value of the agreement with the histological grading by SBR method in comparison to other 3-tier cytological grading systems. Hence, in conclusion, this grading should be routinely incorporated in the cytology reports as it correlates well with histological grade. We wish to caution that FNAC diagnosis and grading of such cases should be done only by a pathologist with reasonable experience in breast cytology, preferably in academic centers where second opinions can readily be obtained. Despite various cytological grading systems, Robinson's method is simple, more objective, and reproducible, hence being preferable for routine use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Robinson's grade. (a) Robinson's Grade I showing monomorphic cell cluster with vesicular nuclei (May-Grunwald Giemsa, ×100). (b) Robinson's Grade II showing mild to moderate pleomorphic tumor cells (May-Grunwald Giemsa, ×400). (c) Robinson's Grade III showing singly scattered cells with marked pleomorphism (H and E, ×100)

| Fig. 2 (a) Mouriquand's Grade III showing large cells with hyperchromatic nuclei and mitosis (H and E, ×400). (b) Fisher's modification of Black's nuclear Grading III showing enlarged tumor cells with prominent nucleoli (H and E, ×400)

| Fig. 3 Nottingham modification of Scarff-Bloom-Richardson grading. (a) Grade I invasive ductal carcinoma, not otherwise specified (H and E, ×100). (b) Grade II invasive ductal carcinoma, not otherwise specified (H and E, ×400). (c) Grade III invasive ductal carcinoma, not otherwise specified (H and E, ×400)


 PDF
PDF  Views
Views  Share
Share

