Comparative Study of Imprint Cytology and Histopathology of Soft Tissue Tumors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 461-465
DOI: DOI: 10.4103/ijmpo.ijmpo_132_16
Abstract
Background: Thecomponents of soft tissue are fibroblasts, collagen, vascular structures, fatty tissue, skeletal muscles, smooth muscles, and neural tissue. The real incidence of soft tissue tumors (STTs) is difficult to estimate because most of them are benign (Benign: Malignant-100:1). Aims: The aim of the present study was undertaken to note the patterns of presentation of patients with STTs and to evaluate the findings of imprint cytology (IC) and histopathological examination (HPE) of STTs. Materials and Methods: The present study was undertaken for 1 year. A total of 41 patients with clinically and radiologically diagnosed STTs were included in the study. Following surgery, imprint smear was taken for each tumor, before delivering the tissue to 10% formalin. HPE was subsequently performed. Results: The age of the patients ranged from 4 months to 80 years with a mean of 35.6 ± 17.5 years. The ratio of males to females was 1.05:1. HPE revealed that 21 (51.2%) tumors were benign and 20 (48.8%) malignant. Imprint smears revealed 16 (39%) tumors to be benign and 20 (48.8%) malignant. IC was inconclusive in 5 (12.2%) cases. The sensitivity of IC was found to be 89.5% and specificity 82.35%. The positive predictive value of IC was 85%. The accuracy of IC for diagnosis of both benign and malignant tumors was found to be 75%. Conclusion: IC of STTs is a rapid and simple method of intraoperative diagnosis, and it can serve as a viable alternative to frozen section biopsy, particularly in rural settings.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The components of soft tissue are fibroblasts, collagen, vascular structures, fatty tissue, skeletal muscles, smooth muscles, and neural tissue. The real incidence of soft tissue tumors (STTs) is difficult to estimate because most of them are benign (Benign: Malignant-100:1).
Aims:
The aim of the present study was undertaken to note the patterns of presentation of patients with STTs and to evaluate the findings of imprint cytology (IC) and histopathological examination (HPE) of STTs.
Materials and Methods:
The present study was undertaken for 1 year. A total of 41 patients with clinically and radiologically diagnosed STTs were included in the study. Following surgery, imprint smear was taken for each tumor, before delivering the tissue to 10% formalin. HPE was subsequently performed.
Results:
The age of the patients ranged from 4 months to 80 years with a mean of 35.6 ± 17.5 years. The ratio of males to females was 1.05:1. HPE revealed that 21 (51.2%) tumors were benign and 20 (48.8%) malignant. Imprint smears revealed 16 (39%) tumors to be benign and 20 (48.8%) malignant. IC was inconclusive in 5 (12.2%) cases. The sensitivity of IC was found to be 89.5% and specificity 82.35%. The positive predictive value of IC was 85%. The accuracy of IC for diagnosis of both benign and malignant tumors was found to be 75%.
Conclusion:
IC of STTs is a rapid and simple method of intraoperative diagnosis, and it can serve as a viable alternative to frozen section biopsy, particularly in rural settings.
Introduction
Soft tissue is defined as tissue that surrounds the epithelial cells and acts as supportive and packing substance. The components are fibroblasts, collagen, vascular structures, fatty tissue, skeletal muscles, and smooth muscles. However, neural tissue which is ectodermal in origin is also included in soft tissue.[1]
Although soft tissue tumors (STTs) are commonly found in surgical practice, its real incidence is difficult to estimate because most of them are benign (Benign: Malignant-100:1). Many of the patients do not seek medical attention, and most of them are not removed.[1] Soft tissue sarcomas constitute 0.7% of all cancers in general population.[2]
The most important aspect of management of STTs is preoperative diagnosis. History, clinical examination, and radiological investigations, particularly computed tomography (CT) and magnetic resonance imaging (MRI) are definitely important in making diagnosis, but pathological preoperative diagnosis remains central to management.[1]
Since excisional biopsy or shelling out of sarcomas is inappropriate and often may cause difficulties in further patient management, it is generally advisable to obtain a diagnostic biopsy (prior to definitive management) for all superficial soft tissue masses >5 cm in greatest dimension (exception being a very obvious subcutaneous lipoma) and for all subfascial or deep-seated masses irrespective of sizes.[3]
The method most routinely used nowadays for preoperative diagnosis of STTs is fine-needle aspiration cytology (FNAC) with several distinct advantages. Numerous studies have endorsed the role of FNAC as an important preoperative tool in making a proper diagnosis which is accurate, cost effective, and well tolerated in trained hands. The accuracy is more when supported by clinical and other diagnostic data, particularly ultrasonography, CT and MRI. All these modalities have improved localization of STTs particularly deep-seated ones.[4]
Touch smear or imprint cytology (IC) prepared from just removed surgical specimens gives excellent cytological clarity and patterns reflecting the architectural orientation of the tumor cells. This is helpful in making an intra-operative diagnosis, and it can also be utilized for evaluating surgical margins which have proved to be relatively simple and cost effective.[5]
The final diagnosis is rendered by histopathological examination (HPE) of the tissue under light microscope in most of the cases. However, in some instances, special techniques (immunohistochemical examination) are required.[6]
This study was undertaken to note the patterns of presentation of patients with STTs and to evaluate the findings of IC and HPE of STTs. It was also attempted to find out if there is any correlation between the results of IC and HPE.
Materials And Methods
The study was undertaken in the Department of Pathology of a tertiary care hospital in Kolkata from June 2014 to May 2015. Patients of all age groups with clinically and radiologically diagnosed STTs were included in the study. Patients with epithelial, visceral, or secondary tumors were excluded from the study.
For each patient, detailed history and clinical findings were noted. Reports of radiological investigations were taken into account. Imprint smear was taken for each tumor, after the operation, before delivering the tissue to 10% formalin. A direct imprint was prepared by pressing a glass slide gently on to the freshly cut surface of the specimen, avoiding any gliding movement, which causes distortion of the shape of cells. Wet-fixed smears were stained with Papanicolaou (PAP) stain and air-dried smears with Leishman–Giemsa (LG) stain.
Formalin fixation was undertaken followed by meticulous grossing of each specimen. Adequate sections were taken from appropriate areas. These were then processed and stained with hematoxylin and eosin stain. Subsequently, HPE was done.
All records were noted and analyzed.
Results
A total of 41 cases were included in the study. The age of the patients ranged from 4 months to 80 years with a mean of 35.6 ± 17.5 years. Males were slightly more affected than females with a male: female ratio of 1.05:1. There were only 4 (9.8%) deep seated STTs found in the study. The other 37 tumors (90.2%) were superficially located.
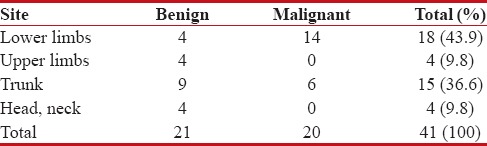
The results of HPE were considered to be the standard against which the accuracy of IC was assessed. HPE revealed that 21 (51.2%) tumors were benign and 20 (48.8%) malignant. The borderline tumors diagnosed by HPE were categorized as malignant for the convenience of comparision with the results of IC. The most common location of malignant tumors was found to be the lower limbs where 14 (70%) tumors were detected. Among the benign tumors, trunk was the most commonly affected site with 9 (42.9%) tumors being situated in it [Table 1].
Table 1
Distribution of soft tissue tumors according to site
|
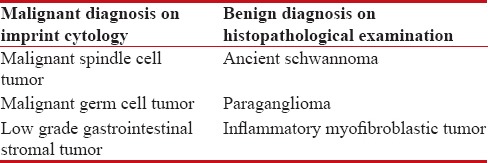
Imprint smears revealed 16 (39%) tumors to be benign and 20 (48.8%) malignant. IC was inconclusive in 5 (12.2%) cases [Table 2]. Discrepancies were found in case of 3 benign and 2 malignant tumors. Comparision of results of IC and HPE is shown in Table 3. The 3 benign tumors in which an erroneous diagnosis of malignancy was rendered from imprint smears included ancient schwannoma, paraganglioma, and inflammatory myofibroblastic tumor. The 2 malignant tumors in which a benign diagnosis was given after an examination of imprint smears were myxoid malignant fibrous histiocytoma (MFH) and primitive neuroectodermal tumor (PNET) [Tables [Tables44 and and55].
Table 2
Results of imprint cytology

|
Table 3
Comparison between results of imprint cytology and histopathological examination

|
Table 4
Benign tumors which were erroneously diagnosed as malignant in imprint smears
|
Table 5
Malignant tumors which were erroneously diagnosed as benign in imprint smears

|
On the basis of the above results, sensitivity of IC was found to be 89.5% and specificity 82.35%. The inconclusive cases were not taken into consideration. The positive predictive value of IC was found to be 85%. P value was significant (0.0001).
Among the 16 benign tumors diagnosed on the basis of IC, specific tumor diagnosis was made in 12 cases. The erroneous diagnosis was made in case of 4 tumors. HPE showed 2 of these tumors to be malignant (myxoid MFH and PNET) and the other 2 benign. The correct specific diagnosis could not be rendered in case of these 2 benign tumors. On the basis of IC, they were diagnosed as cases of nodular fasciitis. Subsequent HPE revealed these tumors to be cases of granulation tissue [Table 6].
Table 6
Accuracy of imprint cytology regarding specific tumor diagnosis
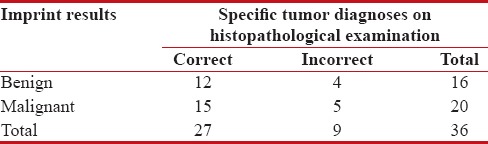
| On the basis of imprint smears, 20 tumors were diagnosed as malignant. HPE showed that specific tumor diagnosis was correctly rendered in 15 cases. The 5 cases in which error was made included 3 benign tumors (ancient schwannoma, paraganglioma, and inflammatory myofibroblastic tumor) and 2 malignant tumors in which specific tumor diagnosis was missed. These 2 cases were diagnosed as fibrosarcoma on the basis of IC. One of them was subsequently diagnosed as dermatofibrosarcoma protruberans and the other as synovial sarcoma, after HPE [Table 6 and Figure 1].
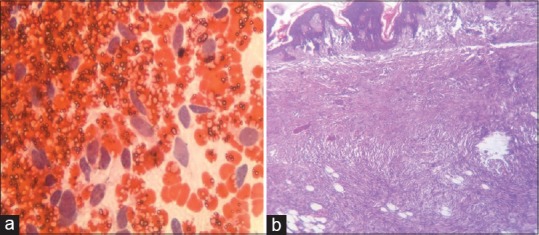
| Figure 1:(a) Imprint smear showing high cellularity with plump slightly pleomorphic spindle cells in haemorrhagic background, suggestive of low grade fibrosarcoma (Papanicolaou, ×400); (b) histologic sections of same tumor showing thin epidermis, homogeneous spindle cells in cart wheel patterna, fat entrapping, absence of tumor giant cells– features were consistent with dermatofibrosarcoma protruberans (H and E, ×100)
Based on the findings of Table 6, the accuracy of IC for diagnosis of both benign and malignant tumors was found to be 75% in the present study [Figures [Figures22 and and33].

| Figure 2:(a) Imprint smear showing high cellularity with strap cells having hyperchromatic nuclei and prominent nucleoli, arranged loosely and singly in a hemorrhagic background with occasional tumor giant cells, suggestive of rhabdomyosarcoma (Leishman–Giemsa, ×400); (b) Histologic sections of the same tumor showing presence of rhabdomyoblasts, confirming the diagnosis of rhabdomyosarcoma (H and E, ×400)
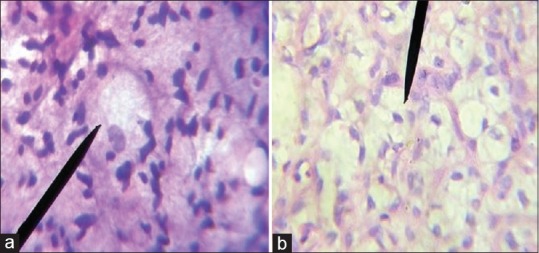
| Figure 3:(a) Imprint smear showing presence of lipoblasts, suggestive of liposarcoma (Papanicolaou ×400); (b) Histologic sections of same tumor showing numerous lipoblasts confirming the diagnosis of liposarcoma (H and E, ×400)
Among the 41 cases included in the study, the surgeon had requested for intraoperative diagnosis of margin status in 18 cases. In 16 cases consistent negative margin status was reported both on IC and HPE. However, IC was incorrect in 2 cases. Subsequent HPE showed that one false positive and another false negative margin were reported based on imprint smears [Table 7]. Based on these results, the specificity of IC for assessing margin status was found to be 94%. However, the sensitivity of IC could not be assessed from the present study since no true positive case encountered.
Table 7
Correlation between margin status reported on imprint smears and histopathological examination

|
Discussion
STTs account for 2%-of all deaths due to malignancies.[1] The incidence in children below 15 years is found to be 15%-and that in adults above 55 years, 40%. The most common site of occurrence of soft tissue sarcomas is the lower extremities.[2]
In this study, the age of the patients ranged between 4 months and 80 years with a mean of 35.6 ± 17.5 years. The ratio of male to female patients was 1.05:1. Colletti et al. in their study of STTs reported a slightly higher mean age of patients (59.9 years) with a range of 12–95 years. They also found a comparable male to female ratio (1.17:1).[7]
Of all the STTs included in the present study, 51.2%-were reported to be benign and 48.8%-malignant. It is extremely difficult to estimate the true incidence of benign and malignant STTs since most benign tumors are asymptomatic and patients do not seek medical attention.[8]
Several studies have reported that IC is an accurate, simple, rapid, and cost effective method of diagnosis. It does not alter the resected specimen which can later be fixed and sectioned.[9,10] Even though frozen section is the most widely used method for intraoperative evaluation of margin status, comparative studies between IC and frozen section have highlighted that the latter is not a practical modality in many situations. This is because frozen sections require expensive instruments, skilled technicians, and histopathologists.[11] Even though architectural orientation is better appreciated in case of frozen sections, artifacts are more commonly encountered. Morphological details are more vivid in imprint smears. The turnaround time is also less in case of imprints.[12]
The diagnostic yield of IC is comparable with that of frozen section. Bhaker et al. reported the diagnostic yield of frozen section to be 90.2%-and that of IC, 87.8%.[12] Ranjan et al. reported a high accuracy rate of IC. They found IC to be 100%-accurate in diagnosing benign and locally infiltrative lesions and 97%-for malignant tumors.[5] Khalid and Haque. showed diagnostic accuracy of both IC and frozen section to be 96.6%-with a sensitivity of 86%-and specificity of 100%.[13]
In this study, the accuracy of IC for diagnosis of both benign and malignant tumors was found to be 75%. The authors of this study enlisted the probable reasons for inaccurate specific diagnoses on the basis of IC. It was found that most benign tumors have low cellular yield and the paucity of cells sometimes leads to the erroneous diagnosis. The cytological picture of some inflammatory lesions closely mimics certain benign or borderline STTs, for example,– granulation tissue was found to mimic nodular fascitis, fibroblasts of granuloma were perceived as spindle cells of fibromatosis. Low-grade sarcomas have little cellularity and mild pleomorphism, and they are commonly diagnosed as benign lesions on IC. Conversely, ancient schwannoma is highly pleomorphic and imprint smears are inadvertently but unavoidably reported as malignant. A case of paraganglioma was missed on IC in the present study. Taweevisit et al. reported that the sustentacular cells that surround the nests of tumor cells in paraganglioma appear as naked nuclei in cytologic smears and they may provide a valuable clue to the diagnosis.[14]
The present study recorded sensitivity of IC to be 89.5%, specificity 82.35%, and positive predictive value 85%. These values were found to be comparable with those reported by Bhaker et al.[12]
Inadequate smears were obtained in 5 (12.2%) cases. The probable reasons for inadequacy were highly cohesive tumor cells, increased fibrosis or sclerosis and excessive necrosis. The disadvantages of IC remain the facts that the tumor cells are required to be at the surface and they must detach themselves from one another to yield an adequate smear.
Conclusion
IC of STTs is a rapid and simple method of intraoperative diagnosis and evaluation of surgical margin status, thus replacing the need for frozen section biopsy, particularly in rural set ups. Even though the present study found the accuracy of imprint diagnosis to be 75%, which is slightly lower than other studies, better results may be obtained by the use of immunocytochemistry, in doubtful cases. Despite its limitations, IC can reliably be considered as a suitable, cheap, and quick mode of diagnosis of STTs. It provides a viable alternative to the frozen section which is expensive, cumbersome, and impossible in most rural areas of developing countries like India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Kumar V, Abbas AK, Aster JC. Bones, joints and soft tissue tumors. In: Kumar V, Abbas AK, Aster JC, editors. Robbins Basic Pathology. 9th ed. Philadelphia, United States: Elsevier Saunders; 2013. p. 765-96.
- Patel SR, Benjamin RS. Soft Tissue and Bone sarcoma and Bone metastases. In: Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed.. USA: McGraw Hill; 2013. p. 817-21.
- Fletcher CD, editor. Tumors of soft tissue. In: Diagnostic Histopathology of Tumors. 4th ed.. Philadelphia, United States: Elsevier Saunders; 2013. p. 1796-870.
- Orell SR, Sterrett GF. Introduction. In: Orell SR, Sterret GF, editors. Orell and Sterrett's Fine Needle Aspiration Cytology. 5th ed.. New Delhi, India: Churchill Livingstone; 2012. p. 1-6.
- Ranjan A, Chandoke RK, Chauhan N, Kumari R. Oncology, study of tumors by imprint cytology. Indian J Clin Pract 2013;24:472-7.
- Iwasaki H, Nabeshima K, Nishio J, Jimi S, Aoki M, Koga K, et al. Pathology of soft-tissue tumors: Daily diagnosis, molecular cytogenetics and experimental approach. Pathol Int 2009;59:501-21.
- Colletti SM, Tranesh GA, Whetsell CR, Chambers LN, Nassar A. High diagnostic accuracy of core needle biopsy of soft tissue tumors: An institutional experience. Diagn Cytopathol 2016;44:291-8.
- Jain P, Shrivastava A, Malik R. Clinicomorphological assessment of soft tissue tumors. Sch J App Med Sci 2014;2:886-90.
- Valdes EK, Boolbol SK, Ali I, Feldman SM, Cohen JM. Intraoperative touch preparation cytology for margin assessment in breast-conservation surgery: Does it work for lobular carcinoma? Ann Surg Oncol 2007;14:2940-5.
- Bakhshandeh M, Tutuncuoglu SO, Fischer G, Masood S. Use of imprint cytology for assessment of surgical margins in lumpectomy specimens of breast cancer patients. Diagn Cytopathol 2007;35:656-9.
- Creager AJ, Shaw JA, Young PR, Geisinger KR. Intraoperative evaluation of lumpectomy margins by imprint cytology with histologic correlation: Acommunity hospital experience. Arch Pathol Lab Med 2002;126:846-8.
- Bhaker P, Mohan H, Handa U, Kumar S. Role of intraoperative pathology consultation in skeletal tumors and tumor-like lesions. Sarcoma 2014;2014:902104.
- Khalid A, Haque AU. Touch impression cytology versus frozen section as intraoperative consultation diagnosis. Int J Pathol 2004;2:63-70.
- Taweevisit M, Bunyayothin W, Thorner PS. Thyroid paraganglioma: “Naked” nuclei as a clue to diagnosis on imprint cytology. Endocr Pathol 2015;26:232-8.

| Figure 1:(a) Imprint smear showing high cellularity with plump slightly pleomorphic spindle cells in haemorrhagic background, suggestive of low grade fibrosarcoma (Papanicolaou, ×400); (b) histologic sections of same tumor showing thin epidermis, homogeneous spindle cells in cart wheel patterna, fat entrapping, absence of tumor giant cells– features were consistent with dermatofibrosarcoma protruberans (H and E, ×100)

| Figure 2:(a) Imprint smear showing high cellularity with strap cells having hyperchromatic nuclei and prominent nucleoli, arranged loosely and singly in a hemorrhagic background with occasional tumor giant cells, suggestive of rhabdomyosarcoma (Leishman–Giemsa, ×400); (b) Histologic sections of the same tumor showing presence of rhabdomyoblasts, confirming the diagnosis of rhabdomyosarcoma (H and E, ×400)

| Figure 3:(a) Imprint smear showing presence of lipoblasts, suggestive of liposarcoma (Papanicolaou ×400); (b) Histologic sections of same tumor showing numerous lipoblasts confirming the diagnosis of liposarcoma (H and E, ×400)
References
- Kumar V, Abbas AK, Aster JC. Bones, joints and soft tissue tumors. In: Kumar V, Abbas AK, Aster JC, editors. Robbins Basic Pathology. 9th ed. Philadelphia, United States: Elsevier Saunders; 2013. p. 765-96.
- Patel SR, Benjamin RS. Soft Tissue and Bone sarcoma and Bone metastases. In: Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed.. USA: McGraw Hill; 2013. p. 817-21.
- Fletcher CD, editor. Tumors of soft tissue. In: Diagnostic Histopathology of Tumors. 4th ed.. Philadelphia, United States: Elsevier Saunders; 2013. p. 1796-870.
- Orell SR, Sterrett GF. Introduction. In: Orell SR, Sterret GF, editors. Orell and Sterrett's Fine Needle Aspiration Cytology. 5th ed.. New Delhi, India: Churchill Livingstone; 2012. p. 1-6.
- Ranjan A, Chandoke RK, Chauhan N, Kumari R. Oncology, study of tumors by imprint cytology. Indian J Clin Pract 2013;24:472-7.
- Iwasaki H, Nabeshima K, Nishio J, Jimi S, Aoki M, Koga K, et al. Pathology of soft-tissue tumors: Daily diagnosis, molecular cytogenetics and experimental approach. Pathol Int 2009;59:501-21.
- Colletti SM, Tranesh GA, Whetsell CR, Chambers LN, Nassar A. High diagnostic accuracy of core needle biopsy of soft tissue tumors: An institutional experience. Diagn Cytopathol 2016;44:291-8.
- Jain P, Shrivastava A, Malik R. Clinicomorphological assessment of soft tissue tumors. Sch J App Med Sci 2014;2:886-90.
- Valdes EK, Boolbol SK, Ali I, Feldman SM, Cohen JM. Intraoperative touch preparation cytology for margin assessment in breast-conservation surgery: Does it work for lobular carcinoma? Ann Surg Oncol 2007;14:2940-5.
- Bakhshandeh M, Tutuncuoglu SO, Fischer G, Masood S. Use of imprint cytology for assessment of surgical margins in lumpectomy specimens of breast cancer patients. Diagn Cytopathol 2007;35:656-9.
- Creager AJ, Shaw JA, Young PR, Geisinger KR. Intraoperative evaluation of lumpectomy margins by imprint cytology with histologic correlation: Acommunity hospital experience. Arch Pathol Lab Med 2002;126:846-8.
- Bhaker P, Mohan H, Handa U, Kumar S. Role of intraoperative pathology consultation in skeletal tumors and tumor-like lesions. Sarcoma 2014;2014:902104.
- Khalid A, Haque AU. Touch impression cytology versus frozen section as intraoperative consultation diagnosis. Int J Pathol 2004;2:63-70.
- Taweevisit M, Bunyayothin W, Thorner PS. Thyroid paraganglioma: “Naked” nuclei as a clue to diagnosis on imprint cytology. Endocr Pathol 2015;26:232-8.


 PDF
PDF  Views
Views  Share
Share

