Comparison of PSA Response to Generic Versus Innovator (Zytiga) Formulations of Abiraterone in Metastatic CRPC: A Retrospective Analysis
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(04): 363-369
DOI: DOI: 10.1055/s-0044-1782235
Abstract
Introduction Abiraterone acetate has been shown to enhance overall survival and radiographic progression-free survival (rPFS) in men with metastatic castration-resistant prostate cancer (mCRPC). Presently, multiple generic brands of abiraterone are accessible in India. Nevertheless, evidence supporting the clinical equivalence of these generics when compared to the innovator has not been established, and thus, questions regarding their quality persist..
Objectives This retrospective analysis aimed to compare the prostate-specific antigen (PSA) response in patients receiving generic or innovator (Zytiga) abiraterone for mCRPC.
Materials and Methods This was a single-center, retrospective, comparative study. All relevant data from selected cases were collected from the hospital's electronic medical record (EMR). Patients with mCRPC, treated with either innovator or generic abiraterone from 2010 to 2019 and followed up until disease progression/death, were included. Patients who switched between generic and reference brands and vice versa were excluded. Patients in both arms were matched for prior treatment with docetaxel (yes/no), age at cancer diagnosis (>60, ≤60 years), and total Gleason's score (≥8, <8>
Results Out of the 114 patients enrolled, 10 patients received Zytiga (innovator), and the remaining received generic abiraterone. No statistically significant difference was observed in the median PSA nadir between the generic and innovator arms: 20.5 versus 88.5 ng/mL (p = 0.293). Patients in the generic group exhibited a similar median rPFS compared to the innovator group: 9.0 months (95% confidence interval [CI]: 6.68–11.31 months) versus 9.0 months (95% CI: 0–18.6 months), respectively (p = 0.539). The median time to PSA nadir was similar (3 months) between the two groups. The proportion of patients showing a PSA response at day 90 did not significantly differ between the two groups, with p = 0.38. The number of adverse events of any grade was comparable between the study groups, although grade 3/4 events were numerically higher in the generic group.
Conclusion Generic abiraterone demonstrates a clinical response similar to that of Zytiga. Our findings strongly support the use of generic abiraterone in patients with mCRPC. The potential economic benefits of this substitution are substantial.
Keywords
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' Contributions
Conception or design of the work was done by V.G., V.N., K.P. and A.J. Data collection was done by A.T., D.V., S.P., and S.K. Data analysis and interpretation were done by S.K. and S.P. S.K. drafted the article. Critical revision of the article was done by V.G., V.N., K.P. and A.J. Final approval of the version to be published was approved by V.G., A.J., D.V., S.P., A.T. and S.K. Accountability for all aspects of the work lies with V.G.
* These authors contributed equally to this work and should be considered as first authors.
Publication History
Article published online:
20 February 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Abiraterone acetate has been shown to enhance overall survival and radiographic progression-free survival (rPFS) in men with metastatic castration-resistant prostate cancer (mCRPC). Presently, multiple generic brands of abiraterone are accessible in India. Nevertheless, evidence supporting the clinical equivalence of these generics when compared to the innovator has not been established, and thus, questions regarding their quality persist..
Objectives This retrospective analysis aimed to compare the prostate-specific antigen (PSA) response in patients receiving generic or innovator (Zytiga) abiraterone for mCRPC.
Materials and Methods This was a single-center, retrospective, comparative study. All relevant data from selected cases were collected from the hospital's electronic medical record (EMR). Patients with mCRPC, treated with either innovator or generic abiraterone from 2010 to 2019 and followed up until disease progression/death, were included. Patients who switched between generic and reference brands and vice versa were excluded. Patients in both arms were matched for prior treatment with docetaxel (yes/no), age at cancer diagnosis (>60, ≤60 years), and total Gleason's score (≥
Results Out of the 114 patients enrolled, 10 patients received Zytiga (innovator), and the remaining received generic abiraterone. No statistically significant difference was observed in the median PSA nadir between the generic and innovator arms: 20.5 versus 88.5 ng/mL (p = 0.293). Patients in the generic group exhibited a similar median rPFS compared to the innovator group: 9.0 months (95% confidence interval [CI]: 6.68–11.31 months) versus 9.0 months (95% CI: 0–18.6 months), respectively (p = 0.539). The median time to PSA nadir was similar (3 months) between the two groups. The proportion of patients showing a PSA response at day 90 did not significantly differ between the two groups, with p = 0.38. The number of adverse events of any grade was comparable between the study groups, although grade 3/4 events were numerically higher in the generic group.
Conclusion Generic abiraterone demonstrates a clinical response similar to that of Zytiga. Our findings strongly support the use of generic abiraterone in patients with mCRPC. The potential economic benefits of this substitution are substantial.
Keywords
Introduction
Prostate cancer is the most commonly diagnosed malignancy in men. Additionally, it is the fifth leading cause of cancer-related death.[1] According to Global Cancer Observatory (GLOBOCAN) reports of 2020, prostate cancer accounts for approximately 7% of cancer incidence and 4% of cancer-related deaths worldwide.[2] Treatment options for early-stage prostate cancer include active surveillance, radical prostatectomy, external beam radiation therapy, brachytherapy, cryotherapy, and androgen deprivation therapy (ADT). Another option is interstitial implantation of radioisotopes such as iodine-125 (125I), palladium, and iridium.[3]
Patients who receive local treatment will experience a biochemical relapse (∼20–40%) and an increased risk of progression to metastasis. Although hormonal therapy has shown effective control of cancer-related symptoms in metastatic prostate cancer, in almost all patients, the disease progresses (resistant to androgen suppression), resulting in a castration-resistant stage.
The various agents used in metastatic castration-resistant prostate cancer (mCRPC) include docetaxel, cabazitaxel, abiraterone, enzalutamide, and apalutamide. Continued ADT (with luteinizing hormone-releasing hormone [LHRH] agonists or antagonists or surgical castration) and abiraterone acetate plus prednisone, docetaxel, or enzalutamide are the standard of care in newly diagnosed mCRPC patients. Abiraterone acetate belongs to the class of antiandrogens and acts by inhibiting CYP17A1 enzyme activity, thus preventing the synthesis of androgen.[4] It has shown to improve survival outcomes in men with mCRPC. A very early prostate-specific antigen (PSA) response (≥50% at 15 days after the start of abiraterone acetate) to abiraterone in mCRPC is a good surrogate marker of progression-free survival (PFS) and overall survival (OS).[5]
The original patent granted for its formulation in 1994 expired in 2014. A controversial secondary patent (the 438 patent) granted in 2014 that covered the coadministration of abiraterone with prednisone for the treatment of CRPC delayed the marketing of generic versions of the drug. However, in October 2018, the U.S. District Court invalidated the 438 patent and generic versions of abiraterone started to appear.[6] Currently, several generic brands of abiraterone are available in India. However, their efficacy has never been established in head-to-head studies with the innovator. In one bioequivalence study between the generic and innovator versions of abiraterone acetate, the generic was found to be bioequivalent to the innovator molecule in healthy adult males.[7] However, such data are not available with most of the generics and hence questions about their quality always remain. One way of establishing clinical equivalence would be to compare the PSA trend in response to abiraterone in patients receiving the generic versus the innovator brands. In this retrospective analysis, we aimed to compare the PSA response in generic versus innovator (Zytiga) abiraterone in metastatic CRPC.
Materials and Methods
Study Design
This was a single-center, retrospective, observational, matched cohort study, conducted in the clinical pharmacology department of a tertiary care cancer hospital in India. The study was approved by the institutional ethics committee (IEC). Pertinent data concerning the selected cases were systematically retrieved from the hospital's electronic medical record (EMR).
Participants
Patients with a confirmed diagnosis of mCRPC treated with either generic or innovator brand of abiraterone from 2010 to 2019 and followed-up till disease progression/death (whichever was earlier) were included in the analysis. Patients who crossed over from generic to innovator brands and vice versa were excluded.
Intervention
The generic formulations of abiraterone investigated in this study included Abirapro 250 mg (Glenmark), Abiratas 250 mg and 500 mg (Intas), Zybiraa 500 mg (Cadila), and Zecyte 250 mg (Cipla). A detailed comparison of pricing between the innovator and generic variations is presented in [Supplementary Table S1] (available in the online version only). Patients were administered oral abiraterone at a dose of 1,000 mg daily along with oral prednisolone at 5 mg twice daily, every 4 weeks according to the standard protocol.
All patient data, encompassing demographics, medical history, treatment history, prostate cancer details (including the date of diagnosis, PSA levels, etc.), baseline symptoms preceding the initiation of abiraterone, baseline laboratory parameters prior to abiraterone commencement, follow-up laboratory investigations, symptoms and adverse events during abiraterone treatment, the cessation date of abiraterone with the reason for discontinuation, the date of progression on abiraterone, specifying the type of progression (biochemical, radiological, clinical), and the date and cause of death, were meticulously documented from the EMR.
Primary and Secondary Outcomes
The primary outcome was to assess the difference in PSA nadir between the innovator and generic cohorts. Secondary outcomes included the evaluation of differences in median radiological PFS (rPFS), median time to nadir, the proportion of patients with a PSA response at 90 days, the incidence of grade ≥3 toxicity, and any-grade toxicity between the two study groups. PSA response was characterized by a reduction in PSA (>50% compared to baseline). Time to nadir was defined as the duration from the initiation of abiraterone to the date of the first observed lowest PSA value. rPFS denoted the time from commencement of abiraterone therapy to radiological disease progression or death from any cause.
Statistical Analysis
All continuous variables were presented as mean and standard deviation or median with interquartile range (IQR). Categorical variables were expressed as proportions (n, %). Differences in means between groups were assessed using Student's t-test, while differences in proportions were evaluated using the chi-squared test/Fisher exact test. Non-normally distributed continuous data (e.g., time to nadir) were compared between groups using the nonparametric Mann–Whitney U test.
The incidence of adverse events was descriptively analyzed and expressed as a percentage. Survival analysis was conducted using the Kaplan–Meier method, and the obtained curves for the innovator and generic groups were compared using a log-rank test. Patients in the innovator and generic arms were matched for disease-free interval (<16>
Data were captured on Microsoft Excel 2019 and analyzed using Statistical Package SPSS v21 and GraphPad Prism 8.0.2. A p-value less than 0.05 was considered statistically significant.
Ethical Approval
The study received approval from the institutional ethics committee (IEC) under the registration number ECR/149/Inst/MH/2013 on September 3, 2021, with project number 900814. Given the retrospective design of the study, a consent waiver was granted by the IEC. All procedures conducted in research involving human participants adhered to ethical standards set by the institutional and/or national research committee, in compliance with the principles outlined in the Declaration of Helsinki (1964) and subsequent amendments or equivalent ethical benchmarks.
Results
A total of 114 eligible patients participated in this study. The patients were categorized based on the abiraterone formulation they received, namely, innovator (n = 10) and generic (n = 104) groups. The final dataset for analyzing all outcomes consisted of 10 patients in the innovator group and 50 patients in the generic group, with the exception of the assessment of PSA response rate (≥50% reduction from baseline) at day 90. For this particular analysis, only 5 of 10 patients in the innovator group and 22 of 50 patients in the generic group were considered evaluable. A schematic illustration of the patient selection process is presented in [Fig. 1]. The baseline demographics of patients who received innovator and generic abiraterone are detailed in [Table 1].
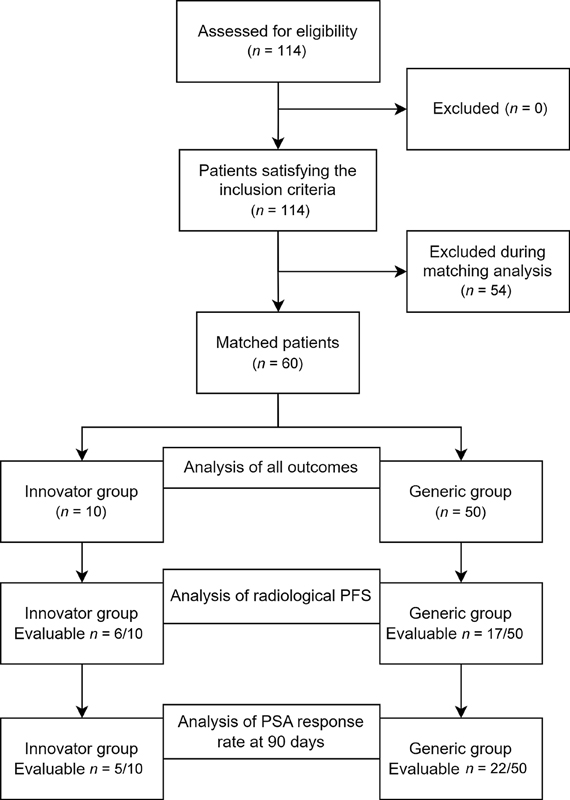
Fig 1: Schematic representation of data collection, matching, and analysis. PFS, progression-free survival; PSA, prostate-specific antigen.
|
Variable |
Innovator (n = 10) |
Generic (n = 50) |
|---|---|---|
|
Age at diagnosis (y), median (IQR) |
64 (53–66) |
62 (55–67) |
|
Smoking (Y/N) |
4/6 |
20/30 |
|
Comorbidities |
||
|
HTN (Y/N) |
3/7 |
26 |
|
DM (Y/N) |
3/7 |
15 |
|
IHD/CAD (Y/N) |
0 |
8 |
|
Initial PSA (ng/mL), median (IQR) |
149 (60–963.5) |
91.8 (27–365.0) |
|
Serum testosterone level (ng/dL), median (IQR) |
8.06 (5.60–26.33) |
16.3 (8.90–21.80) |
|
Initial Gleason's score |
||
|
3 + 2 |
– |
1 |
|
3 + 3 |
3 |
1 |
|
3 + 4 |
1 |
2 |
|
4 + 3 |
2 |
9 |
|
3 + 5 |
– |
1 |
|
4 + 4 |
2 |
18 |
|
4 + 5 |
2 |
11 |
|
5 + 4 |
– |
4 |
|
3 + 7 |
– |
1 |
|
5 + 5 |
– |
2 |
|
Stage at diagnosis (IV/Localized) |
9/1 |
44/6 |
|
Previous definitive surgery (Y/N) |
2/8 |
16 |
|
Previous definitive radiotherapy (Y/N) |
1/9 |
4 |
|
Neoadjuvant HT (Y/N) |
1/9 |
6 |
|
Previous HT (Y/N) |
8/2 |
41 |
|
Type of ADT received |
||
|
Not received |
– |
1 |
|
Surgical |
5 |
29 |
|
Medical |
3 |
16 |
|
Surgical and medical |
2 |
4 |
|
Site of metastasis |
||
|
Bone |
4 |
27 |
|
Lymph node |
– |
3 |
|
Bone, lymph node |
4 |
18 |
|
Bone, lymph node, liver |
1 |
– |
|
Bone, lung |
1 |
– |
|
Bone, lymph node, lung |
– |
2 |
|
Matched factors |
||
|
Disease-free interval (<16> |
4/6 |
20/30 |
|
ECOG-PS (1/2) |
8/2 |
40/10 |
|
Previous therapy with docetaxel/cabazitaxel (Y/N) |
7/3 |
35/15 |
The median PSA nadir in the generic cohort was 20.5 ng/mL (IQR = 3–153.8 ng/mL), contrasting with 88.5 ng/mL (IQR = 25–145.8 ng/mL) in the innovator cohort (p = 0.131; Mann–Whitney U test). rPFS was computed for patients administered with innovator and generic abiraterone. A comparison of median rPFS revealed no significant difference between the groups, as depicted in [Fig. 2]. The generic group exhibited a comparable median rPFS to the innovator group: 9.0 months (95% CI, 6.68–11.31) vs. 9.0 months (95% CI, 0–18.6), p = 0.539. The median (IQR) time to PSA nadir demonstrated no significant difference between the generic and innovator groups, with values of 3 months (IQR = 2–5 months) and 3 months (IQR = 1–3 months), respectively (p = 0.304), as illustrated in [Fig. 3]. Only 5 of 10 patients in the innovator group and 22 of 50 patients in the generic group were evaluable to assess PSA response (≥50% reduction from baseline) at day 90. The proportion of patients exhibiting PSA response at day 90 did not differ significantly between the two groups, as outlined in [Table 2]. The incidence and severity of adverse events in the generic and innovator groups were comparable as shown in [Table 3].
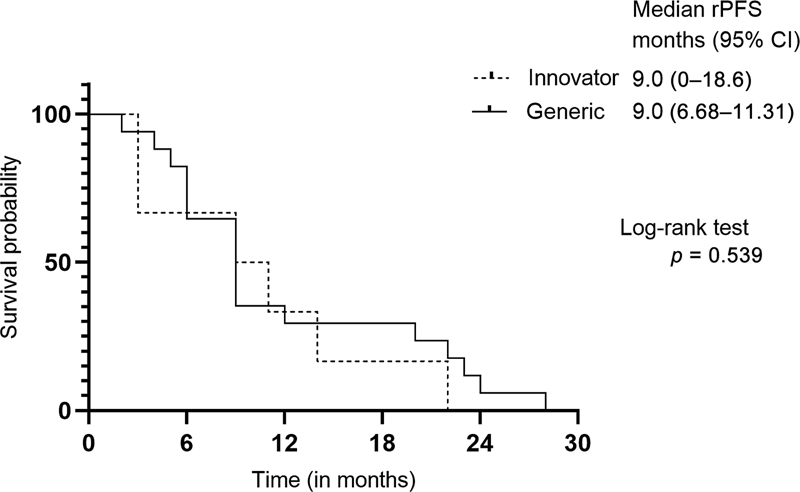
Fig 2 : Progression-free survival estimates between the groups determined using the Kaplan–Meier method. CI, confidence interval; rPFS, radiographic progression-free survival.
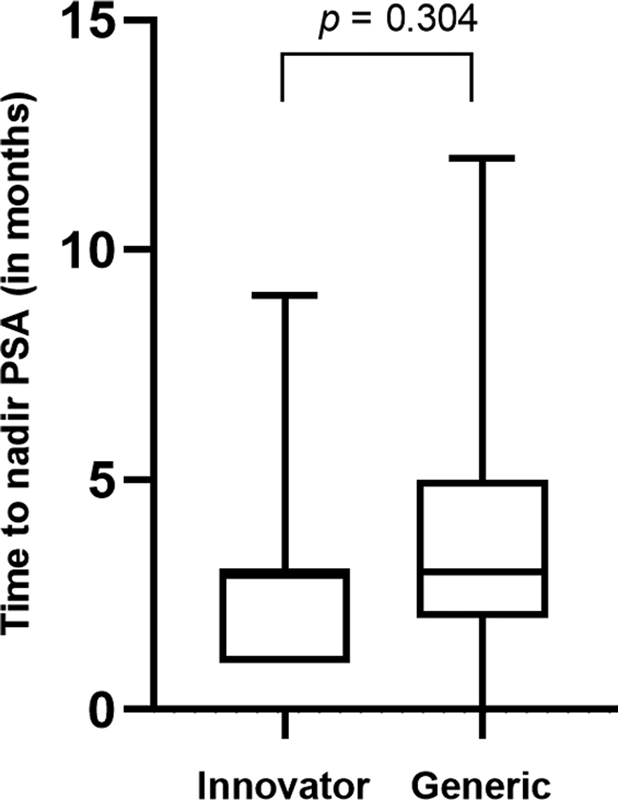
Fig 3 : Difference in time to nadir between innovator and treatment groups. PSA, prostate-specific antigen.
|
Innovator, n (%) Evaluable (n = 5/10) |
Generic, n (%) Evaluable (n = 22/50) |
p-value[a] |
||
|---|---|---|---|---|
|
PSA response at 90 (≥50%) |
Yes |
1 (20) |
9 (40.90) |
0.621 |
|
No |
4 (80) |
13 (59.09) |
|
Adverse event |
Any grade |
Grade 3/4 |
||
|---|---|---|---|---|
|
Innovator |
Generic |
Innovator |
Generic |
|
|
No. of patients with event (%.) |
||||
|
Any adverse event |
7 (70) |
42 (84) |
1 (10) |
3 (6) |
|
Anemia |
4 (40) |
28 (56) |
1 (10) |
2 (4) |
|
Thrombocytopenia |
0 (0) |
4 (8) |
0 (0) |
1 (2) |
|
Neutropenia |
0 (0) |
1 (2) |
0 (0) |
0 (0) |
|
Nausea |
2 (20) |
5 (10) |
0 (0) |
0 (0) |
|
Vomiting |
0 (0) |
6 (12) |
0 (0) |
1 (2) |
|
Deranged LFT |
2 (20) |
8 (16) |
0 (0) |
0 (0) |
|
Rise in bilirubin |
0 (0) |
3 (6) |
0 (0) |
0 (0) |
|
Rise in SGOT |
2 (20) |
5 (10) |
0 (0) |
0 (0) |
|
Rise in SGPT |
2 (20) |
4 (8) |
0 (0) |
0 (0) |
|
Hypophosphatemia |
0 (0) |
7 (14) |
0 (0) |
0 (0) |
|
Hypernatremia |
0 (0) |
1 (2) |
0 (0) |
0 (0) |
|
Fluid retention |
0 (0) |
2 (4) |
0 (0) |
0 (0) |
|
Cardiac disorder |
1 (10) |
3 (6) |
0 (0) |
0 (0) |
|
Hypokalemia |
1 (10) |
4 (8) |
0 (0) |
0 (0) |
|
Fatigue |
2 (20) |
10 (20) |
0 (0) |
1 (2) |
|
Hypertension |
3 (30) |
20 (40) |
0 (0) |
0 (0) |
Discussion
It was hypothesized that the PSA response would exhibit similarity among patients administered generic and innovator (Zytiga) abiraterone formulations. The findings indicated that nadir PSA values, time to PSA nadir, rPFS, PSA response rate at 90 days, and the incidence of adverse events were comparable between the investigated cohorts. U.S. Food and Drug Administration (FDA) defines a generic drug as “a drug product that is comparable to a brand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use.”[8] Previous reports highlighting substandard quality in Indian generics for drugs like docetaxel and L-asparaginase raise concerns.[9] [10] [11] Despite 13 generic brands of abiraterone being approved in India, their clinical equivalence to Zytiga remains uncertain. Wang et al previously demonstrated the bioequivalence of a Chinese abiraterone brand with Zytiga in a single-dose, open-label, replicate design study.[7] Given the absence of bioavailability or bioequivalence data for any of the Indian generics, this retrospective study was designed to assess their clinical equivalence to Zytiga.
PSA kinetics, including PSA nadir, PSA response rates (≥30, 50, and 90%.), time to PSA progression, and PSA doubling time (PSADT), serve as well-established surrogate endpoints indicative of clinical benefit in patients with mCRPC undergoing abiraterone treatment, irrespective of chemotherapy administration.[12] Nadir PSA level refers to the lowest PSA level following primary androgen deprivation therapy. No significant difference in the median nadir PSA levels was observed between the innovator and generic cohorts. Within the generic group, 26%. (13 of 50 patients) exhibited a PSA nadir value of less than 4 ng/mL, while none of the patients in the innovator group displayed such values. The mean time to PSA nadir with abiraterone treatment is reported to be 18.8 ± 9.1 weeks.[13] Nakayama et al reported a similar time to PSA nadir (15.5 weeks) among abiraterone responders in chemotherapy-treated mCRPC patients.[14] However, our observation indicated a marginally earlier median time to PSA nadir, specifically 3 months or 12 weeks, in both study groups.
The median rPFS in both the innovator and generic abiraterone groups demonstrated similarity (9 months), surpassing existing evidence. The COU-AA-301 study reported a median rPFS of 5.6 months with abiraterone treatment (95%. CI, 5.6–6.5 months).[15] Real-world data analysis of Australian mCRPC patients treated with abiraterone also reported an rPFS of 5 months (95%. CI, 2.0–8.0 months).[16] It is known that patients with mCRPC achieving ≥50%. decline in PSA from baseline exhibit improved survival compared to nonresponders.[17] Between study groups, no statistically significant difference in PSA response rates (≥50%. decline in PSA at 90 days from baseline) was observed (p = 0.38). In the generic group, 40%. of patients (9/22) achieved greater than 50%. decline in PSA from baseline at day 90, while only 1/5 evaluable patients in the innovator group reached this threshold. Remaining evaluable patients in the generic group experienced early progression (PFS: 3–5 months) with no observable declining trend in PSA. These generic group findings align with prior reports indicating that approximately 40%. of patients achieve ≥50%. decline in PSA at 90 days during abiraterone treatment.[5] [18] Adverse events of any grade were comparable between study groups, indicating generic formulations did not pose heightened safety concerns, although grade 3/4 events were numerically higher in the generic group.
Overall, these findings suggest that generic abiraterone is clinically equivalent to the innovator. The government is currently emphasizing the potential economic benefits associated with the use of generic drugs. Indurlal et al demonstrated that therapeutic interchange from brand to generic abiraterone leads to substantial savings for the Oncology Care Model in the United States.[19] Additionally, utilizing generic drugs may contribute to savings in drug expenditure. Meanwhile, concerns about the quality and equivalence of generic drugs are increasingly discussed in the lay media.
This study had a few limitations due to its retrospective design, including potential residual confounding stemming from unobserved characteristics of the formulation, selection bias, the inability to assess all available generic versions of abiraterone, the absence of a cost-effectiveness analysis, and a limited number of patients in the innovator group. Additionally, grouping the generic brands together precluded the identification of any distinctions between them. Despite these inherent biases, it is noteworthy that PSA nadir numerically favored generics over the innovator. The limited number of patients in the innovator group may have contributed to an underestimation of both the PSA response rate and adverse events. Our assessment focused on the outcome of the “number of patients having ≥50%. decline in PSA at 90 days from baseline.” However, recent studies have suggested that a greater than 30%. decline in PSA at 4 weeks[20] and a ≥50%. decline in PSA at 15 days[5] could be valuable in identifying patients unlikely to benefit from abiraterone, serving as surrogates for longer PFS and OS, respectively. The incorporation of these metrics might have underscored the contemporary utility of generic brands. Nevertheless, it is unlikely that the outcomes would have been substantially different.
Conclusion
In this small retrospective analysis, the clinical outcomes observed with generic abiraterone were found to be comparable to those associated with Zytiga. Our study provides evidence endorsing the utilization of generic abiraterone among patients diagnosed with mCRPC, particularly in scenarios where access to Zytiga is limited. The substantial advantages of prescribing generic abiraterone for individuals with mCRPC are noteworthy.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank the patients and their families for participating in this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' Contributions
Conception or design of the work was done by V.G., V.N., K.P. and A.J. Data collection was done by A.T., D.V., S.P., and S.K. Data analysis and interpretation were done by S.K. and S.P. S.K. drafted the article. Critical revision of the article was done by V.G., V.N., K.P. and A.J. Final approval of the version to be published was approved by V.G., A.J., D.V., S.P., A.T. and S.K. Accountability for all aspects of the work lies with V.G.
* These authors contributed equally to this work and should be considered as first authors.
Supplementary Material
Supplementary MaterialReferences
- Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health 2022; 10: 811044
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- PDQ Adult Treatment Editorial Board. Prostate Cancer Treatment (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute (US); 2002
- Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther 2012; 6: 13-18
- Facchini G, Caffo O, Ortega C. et al. Very early PSA response to abiraterone in mCRPC patients: a novel prognostic factor predicting overall survival. Front Pharmacol 2016; 7: 123
- Miller D, Ippolito B, Hernandez I, Davies B. The costs of delayed generic drug entry: evidence from a controversial prostate cancer drug patent. J Gen Intern Med 2022; 37 (03) 668-670
- Wang C, Hu C, Gao D. et al. Pharmacokinetics and bioequivalence of generic and branded abiraterone acetate tablet: a single-dose, open-label, and replicate designed study in healthy Chinese male volunteers. Cancer Chemother Pharmacol 2019; 83 (03) 509-517
- Meredith PA. Generic drugs. Drug-Safety 1996; 15: 233-242
- Sankaran H, Sengupta S, Purohit V. et al. A comparison of asparaginase activity in generic formulations of E. coli derived L-asparaginase: in-vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br J Clin Pharmacol 2020; 86 (06) 1081-1088
- Johnson S, Dhamne C, Sankaran H. et al. A prospective, open-label, randomised, parallel design study of 4 generic formulations of intramuscular L-asparaginase in childhood precursor B-cell acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol 2022; 90 (06) 445-453
- Vial J, Cohen M, Sassiat P, Thiébaut D. Pharmaceutical quality of docetaxel generics versus originator drug product: a comparative analysis. Curr Med Res Opin 2008; 24 (07) 2019-2033
- Xu XS, Ryan CJ, Stuyckens K. et al. Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res 2015; 21 (14) 3170-3177
- Miyake H, Hara T, Tamura K. et al. Independent association between time to prostate-specific antigen (PSA) nadir and PSA progression-free survival in patients with docetaxel-naïve, metastatic castration-resistant prostate cancer receiving abiraterone acetate, but not enzalutamide. Urol Oncol 2017; 35 (06) 432-437
- Nakayama M, Kobayashi H, Takahara T, Oyama R, Imanaka K, Yoshizawa K. Association of early PSA decline and time to PSA progression in abiraterone acetate-treated metastatic castration-resistant prostate cancer; a post-hoc analysis of Japanese phase 2 trials. BMC Urol 2016; 16 (01) 27
- Fizazi K, Scher HI, Molina A. et al; COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13 (10) 983-992
- Raju R, Sahu A, Klevansky M, Torres J. Real-world data on outcomes in metastatic castrate-resistant prostate cancer patients treated with abiraterone or enzalutamide: a regional experience. Front Oncol 2021; 11: 656146
- Halabi S, Armstrong AJ, Sartor O. et al. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J Clin Oncol 2013; 31 (31) 3944-3950
- Demirci A, Bilir C, Gülbağcı B. et al. Comparison of real-life data of abiraterone acetate and enzalutamide in metastatic castration-resistant prostate cancer. Sci Rep 2021; 11 (01) 14131
- Indurlal P, Ives H, Garey JS, McGuinness M, Pacheco AV, Wilfong LS. Financial impact of generic therapeutic interchange of abiraterone in the oncology care model for the U.S. Oncology Network. J Clin Oncol 2022; 40 (16, suppl): e17019-e17019
- Rescigno P, Lorente D, Ferraldeschi R. et al. Association between PSA declines at 4 weeks and OS in patients treated with abiraterone acetate (AA) for metastatic castration resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol 2015; 33 (7, suppl): 215-215
Address for correspondence
Publication History
20 February 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Clinicoradiological Profile and Treatment Outcomes in Prostate Cancer at a Tertiary Care Cancer Center in IndiaAstha Rajput, Indian Journal of Medical and Paediatric Oncology, 2020
- Improving quality of life in patients with metastatic prostate cancer following one cycle of 177Lu-PSMA-617 radioligand therapy: a pilot studyMilka Marinova, Nuklearmedizin
- Improving quality of life in patients with metastatic prostate cancer following one cycle of 177Lu-PSMA-617 radioligand therapy: a pilot studyMilka Marinova, Zentralblatt für Chirurgie - Zeitschrift für Allgemeine, Viszeral-, Thorax- und Gefäßchirurgie
- Report of chronic myeloid leukemia in chronic phase from Tata Memorial Hospital, Mumbai, 2002-2008Purvish Parikh, Indian Journal of Medical and Paediatric Oncology, 2013
- Innovator Filgrastim versus Generic Filgrastim in Hematopoietic Stem Cell Transplantation MobilizationSadik Husian, et al., South Asian Journal of Cancer, 2021
- Generic personal safety applications: empowering victims of domestic violence and abuse? A practitioner lensDi Turgoose, Journal of Gender-Based Violence, 2021
- Research note: A prenotice greeting card’s impact on response rates and response timeAshley K. Griggs, Longitudinal and Life Course Studies, 2019
- Clinical Decision Support Systems for Outcome Measurement and ManagementSakallaris, AACN Adv Crit Care, 2000
- Acute Respiratory Distress Syndrome Prediction Score: Derivation and ValidationLixue Huang, Am J Crit Care, 2021
- Intensity and spatial extension of drought in South Africa at different time scalesMathieu Rouault, IFE PsychologIA : An International Journal

Fig 1: Schematic representation of data collection, matching, and analysis. PFS, progression-free survival; PSA, prostate-specific antigen.

Fig 2 : Progression-free survival estimates between the groups determined using the Kaplan–Meier method. CI, confidence interval; rPFS, radiographic progression-free survival.

Fig 3 : Difference in time to nadir between innovator and treatment groups. PSA, prostate-specific antigen.
References
- Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health 2022; 10: 811044
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- PDQ Adult Treatment Editorial Board. Prostate Cancer Treatment (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute (US); 2002
- Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther 2012; 6: 13-18
- Facchini G, Caffo O, Ortega C. et al. Very early PSA response to abiraterone in mCRPC patients: a novel prognostic factor predicting overall survival. Front Pharmacol 2016; 7: 123
- Miller D, Ippolito B, Hernandez I, Davies B. The costs of delayed generic drug entry: evidence from a controversial prostate cancer drug patent. J Gen Intern Med 2022; 37 (03) 668-670
- Wang C, Hu C, Gao D. et al. Pharmacokinetics and bioequivalence of generic and branded abiraterone acetate tablet: a single-dose, open-label, and replicate designed study in healthy Chinese male volunteers. Cancer Chemother Pharmacol 2019; 83 (03) 509-517
- Meredith PA. Generic drugs. Drug-Safety 1996; 15: 233-242
- Sankaran H, Sengupta S, Purohit V. et al. A comparison of asparaginase activity in generic formulations of E. coli derived L-asparaginase: in-vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br J Clin Pharmacol 2020; 86 (06) 1081-1088
- Johnson S, Dhamne C, Sankaran H. et al. A prospective, open-label, randomised, parallel design study of 4 generic formulations of intramuscular L-asparaginase in childhood precursor B-cell acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol 2022; 90 (06) 445-453
- Vial J, Cohen M, Sassiat P, Thiébaut D. Pharmaceutical quality of docetaxel generics versus originator drug product: a comparative analysis. Curr Med Res Opin 2008; 24 (07) 2019-2033
- Xu XS, Ryan CJ, Stuyckens K. et al. Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res 2015; 21 (14) 3170-3177
- Miyake H, Hara T, Tamura K. et al. Independent association between time to prostate-specific antigen (PSA) nadir and PSA progression-free survival in patients with docetaxel-naïve, metastatic castration-resistant prostate cancer receiving abiraterone acetate, but not enzalutamide. Urol Oncol 2017; 35 (06) 432-437
- Nakayama M, Kobayashi H, Takahara T, Oyama R, Imanaka K, Yoshizawa K. Association of early PSA decline and time to PSA progression in abiraterone acetate-treated metastatic castration-resistant prostate cancer; a post-hoc analysis of Japanese phase 2 trials. BMC Urol 2016; 16 (01) 27
- Fizazi K, Scher HI, Molina A. et al; COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13 (10) 983-992
- Raju R, Sahu A, Klevansky M, Torres J. Real-world data on outcomes in metastatic castrate-resistant prostate cancer patients treated with abiraterone or enzalutamide: a regional experience. Front Oncol 2021; 11: 656146
- Halabi S, Armstrong AJ, Sartor O. et al. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J Clin Oncol 2013; 31 (31) 3944-3950
- Demirci A, Bilir C, Gülbağcı B. et al. Comparison of real-life data of abiraterone acetate and enzalutamide in metastatic castration-resistant prostate cancer. Sci Rep 2021; 11 (01) 14131
- Indurlal P, Ives H, Garey JS, McGuinness M, Pacheco AV, Wilfong LS. Financial impact of generic therapeutic interchange of abiraterone in the oncology care model for the U.S. Oncology Network. J Clin Oncol 2022; 40 (16, suppl): e17019-e17019
- Rescigno P, Lorente D, Ferraldeschi R. et al. Association between PSA declines at 4 weeks and OS in patients treated with abiraterone acetate (AA) for metastatic castration resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol 2015; 33 (7, suppl): 215-215


 PDF
PDF  Views
Views  Share
Share

