Comprehensive Germline Genomic Profiling of Patients with Ovarian Cancer: A Cross-Sectional Study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(04): 361-368
DOI: DOI: 10.1055/s-0042-1746197
Abstract
Introduction Ovarian cancer is the third most common cancer among Indian women. The data on the hereditary predisposition of these cancers and the clinical outcomes of those with pathogenic mutations is meager in India.
Objective The aim of the current study was to analyze the germline-genetic profile, clinicopathological characteristics, and outcomes of patients with ovarian cancer who were referred for genetic counseling at our Institute.
Materials and Methods It was a cross-sectional observational study. Patients with histological diagnosis of carcinoma ovary at our institute who were referred for genetic counseling from July 2017 to June 2020 were included in the study. All patients underwent pretest counseling. Most patients underwent multigene panel testing with reflex multiplication ligation-dependent probe amplification for large genomic rearrangements, while some received testing for BRCA1 and BRCA2 only. The variants were classified as pathogenic or benign based on American College of Medical Genetics (ACMG) guidelines. Data regarding the demographic profile, clinical characteristics, histopathological findings, family history, treatment received, and outcomes were extracted from the medical record system files.
Results One hundred and one patients were referred to the genetic clinic and underwent genetic counseling. All patients were advised for genetic testing; however, only 72 (71%) underwent testing. A multigene panel testing was done in 51 (70%) patients, and only BRCA1 and BRCA2 genes were tested in 21 (30%). Among the 72 patients who underwent a genetic test, the median age was 47 years (range, 28–82). The most common histopathology was serous (90%), while 85% were diagnosed having stage 3 and 4 ovarian cancer. A pathogenic/likely pathogenic (P/LP) BRCA or non-BRCA mutation was detected in 32 (44%) patients. Six patients (8%) had a variant of unknown significance (VUS). Among P/LP mutations, 85% were in the BRCA gene (75% in BRCA1 and 10% in BRCA2), while 15% were in non-BRCA gene mutations (RAD51, PALB2, MER11, HMMR). Disease-free survival and overall survival were not different in mutation-positive and mutation-negative cohorts.
Conclusions We report 44% P/LP mutations in this selected cohort of patients with carcinoma ovaries. BRCA mutations constituted 85% of all the mutations, while 15% of mutations were in non-BRCA genes.
Keywords
germline - ovarian cancer - BRCAEthical Approval
The study protocol was approved by the Institute Ethics Committee vide letter number-IEC-511/5.6.20 RP/50/2020
Consent to Participate
Informed consent was obtained from all patients.
Availability of Data and Material
Data regarding this study will be available from the corresponding author (RP) at reasonable request.
Authors' Contributions
R.P; A.U; S.K; L.K; P.M; M.R; S.D; and S.K contributed to the concept design, patient referrals, and conduct of the study. R.G; D.T; and V.L.R were involved in laboratory testing. A.U; R.P did the statistical analysis.
Supplementary MaterialPublication History
Article published online:
01 September 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Ovarian cancer is the third most common cancer among Indian women. The data on the hereditary predisposition of these cancers and the clinical outcomes of those with pathogenic mutations is meager in India.
Objective The aim of the current study was to analyze the germline-genetic profile, clinicopathological characteristics, and outcomes of patients with ovarian cancer who were referred for genetic counseling at our Institute.
Materials and Methods It was a cross-sectional observational study. Patients with histological diagnosis of carcinoma ovary at our institute who were referred for genetic counseling from July 2017 to June 2020 were included in the study. All patients underwent pretest counseling. Most patients underwent multigene panel testing with reflex multiplication ligation-dependent probe amplification for large genomic rearrangements, while some received testing for BRCA1 and BRCA2 only. The variants were classified as pathogenic or benign based on American College of Medical Genetics (ACMG) guidelines. Data regarding the demographic profile, clinical characteristics, histopathological findings, family history, treatment received, and outcomes were extracted from the medical record system files.
Results One hundred and one patients were referred to the genetic clinic and underwent genetic counseling. All patients were advised for genetic testing; however, only 72 (71%) underwent testing. A multigene panel testing was done in 51 (70%) patients, and only BRCA1 and BRCA2 genes were tested in 21 (30%). Among the 72 patients who underwent a genetic test, the median age was 47 years (range, 28–82). The most common histopathology was serous (90%), while 85% were diagnosed having stage 3 and 4 ovarian cancer. A pathogenic/likely pathogenic (P/LP) BRCA or non-BRCA mutation was detected in 32 (44%) patients. Six patients (8%) had a variant of unknown significance (VUS). Among P/LP mutations, 85% were in the BRCA gene (75% in BRCA1 and 10% in BRCA2), while 15% were in non-BRCA gene mutations (RAD51, PALB2, MER11, HMMR). Disease-free survival and overall survival were not different in mutation-positive and mutation-negative cohorts.
Conclusions We report 44% P/LP mutations in this selected cohort of patients with carcinoma ovaries. BRCA mutations constituted 85% of all the mutations, while 15% of mutations were in non-BRCA genes.
Introduction
Ovarian cancer (OC) is the eighth most common cancer in females (3.4% of all cancers) and leads to 4.4% of all cancer-related mortality.[1] Globally, there were 313,959 cases diagnosed with OC in 2020 and 207,252 deaths.[1] In India, the estimated incidence of OC in 2020 is 43,886 (6.7%) cases, and mortality is 32,077 (7.8%).[2] Though the prevalence is low, the survival of advanced OCs is dismal (20–40%).[3] The most important risk factor for OC is a genetic predisposition, that is, family history of breast or OC.[4] More than 20% of OCs are associated with a mutation in the tumor suppressor genes, most important being BRCA1/2.[5] The average cumulative frequency of OC at 70 years is estimated to be 35 to 46% for individuals with BRCA1 mutations and 13 to 23% for BRCA2 mutation.[6] Other genes implicated are ATM, CHEK2, BRIP, RAD51C, RAD51D, and MUTYH genes.[5]
Families with these mutations have a higher prevalence of multiple malignancies. Preventive strategies like salpingo-oophorectomy reduce the risk of OC by 96%.[7] Detecting mutation in the BRCA gene has therapeutic implications and is immediately applicable to patients in the first line as maintenance and in the recurrent setting where it has shown improvements in progression-free survival.[8] [9] Identification of these pathogenic mutations is essential as this may help us plan screening and preventive strategies in families with identifiable mutations. The current literature regarding mutation-positive OC in India is meager and is often lumped with breast cancer data. The current study aimed to analyze the genetic and clinicopathological profile and outcomes of patients with OC from a tertiary care center in India.
Methodology
Materials and Methods
Patients
In this cross-sectional observational study, patients with histological diagnosis of carcinoma of the ovary, registered in our cancer center, who were referred for genetic counseling between Jan 2017 and Dec 2020 were included. A medical oncologist did pretest counseling. Patients who did not give informed consent were excluded. Most patients underwent multigene panel testing with reflex multiplication ligation-dependent probe amplification (MLPA) that detects gene specific single or multiple exon deletions and duplications, which may be missed by next-generation sequencing, while some received testing for BRCA1 and BRCA2 only. Those with a pathogenic/likely pathogenic (P/LP) mutation in any of the predisposing genes were discussed in a multidisciplinary tumor board for management and were offered post-test counseling. The variants were classified as pathogenic or benign based on American College of Medical Genetics (ACMG) guidelines.[10] Data regarding the demographic profile, clinical characteristics, histopathological findings, family history, treatment received, and outcomes were extracted from the medical record system files. The primary outcome was the frequency of PLA mutations identified in the sample. The secondary outcomes included the comparison of response rates, overall survival (OS), and disease-free survival between the groups with and without a P/LP mutation.
Sample Preparation and Multigene Panel Design
Blood samples were collected from each patient (10 mL), and germline DNA extraction was done by kits following the manufacturer's instructions. Genomic DNA was enzymatically fragmented. Regions of interest were selectively enriched using customed capture probes targeted against coding regions of the 104 genes (listed in [supplementary material]), including ten bp of flanking intronic sequences for the genes BRCA1 and BRCA2. In 21 patients, only two genes (BRCA1 and BRCA2) were studied. The libraries underwent next-generation sequencing to mean >80–100X coverage on the Illumina sequencing platform. The sequences were aligned to the human reference genome (GRCh37/hg19) build I.D. Gene bank NM_007300.3 and NM _000059.3 were used as reference transcript sequences for BRCA1 and BRCA2 genes, respectively. Gene annotation of the variants was performed using the variant effect predictor (VEP) program against Ensemble release 91 human gene model. Mutations were annotated using databases: ClinVar, OMIM, GWAS, HGMD, and SwissVar. Variants were filtered based on allele frequency in 1000 Genome phase3, gnomAD, EVS, dbSNP, 1000 Japanese Genome, and Indian database.
MLPA Testing
Reflex MLPA testing was done for patients who tested negative on the multigene panel. Copy number change in 24 exons of BRCA1 and 27 exons of BRCA2 was identified by hybridizing with MLPA-based assay. Each MLPA probe consisted of two hemiprobes that were bound to an adjacent site of the target sequence. Upon ligation and subsequent PCR amplification, each probe generated an amplicon with a unique length. Copy number differences of various exons between test and control DNA samples were detected by analyzing the MLPA peak patterns. The classification of variants was done as deleterious (class 5—pathogenic or class 4—likely pathogenic), a variant of unknown clinical significance (VUS, class 3), likely benign (class 2), and benign (class 1) according to the ACMG guidelines.
Data regarding the demographic profile, clinical characteristics, histopathological findings, family history, treatment received, and outcomes were extracted from the medical record system files.
Statistical Analysis
Categorical data were summarized using percentages. Numerical data were summarized as the means and standard deviations or medians and ranges. Chi-squared tests and Fisher's exact tests were used to examine the relationships between qualitative variables. The survival analysis was performed using the Kaplan–Meier method. A log-rank test was used to compare the survival curves. All tests of hypotheses were conducted at an α level of 0.05, with a 95% -confidence interval. The median duration of follow-up was estimated using the reverse Kaplan–Meier method. Disease-free survival) was defined as “the interval from histological diagnosis to the date of first progression or last follow-up.” OS was defined as “the interval from histological diagnosis to the date of death or last follow-up.” All analysis was done using STATA software (ver13, Texas, United States)
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. Ethics Committee Approval was obtained from the Institutional Ethics Committee vide letter no. IEC-511/05.06.2020, RP-50/2020 dated 30.9.2020 (see [supplementary material]).
Results
One hundred and one patients were referred to the genetic clinic and underwent genetic counseling. All patients were advised for genetic testing; however, only 72 of them (71%) underwent testing. The various reasons for not undergoing genetic testing were financial issues in 20 patients (20%), deferral of testing due to COVID pandemic in 5 of them (5%), and patient preference in 4 (5%). A multigene panel testing was done in 51 (70%) patients, and only BRCA1 and BRCA2 genes were tested in 21 (30%).
Among the 72 patients who underwent a genetic test, the median age was 47 years (range 28–82) ([Table 1]). Hinduism was the most common religion (89%), while four (5%) patients were Muslims and two (3%) were Christians. Seventy-three percent of these patients were literate. Seventy-four percent of these patients were from Delhi, Chandigarh, and Uttar Pradesh, while 18% were from eastern states (Bihar, Orissa, West Bengal). Six patients were from western and southern states (four from Rajasthan and two from Kerala).
|
Baseline characteristics(n = 72) |
Median/mean ± SD/percentage |
|---|---|
|
Age |
45.5 years (IQR: 41.5–55) (Range, 28–82) |
|
Ethnicity |
|
|
Hindu |
65 (90%) |
|
Muslim |
04 (5%) |
|
Christian |
02 (3%) |
|
Buddhism |
01 (2%) |
|
Histology |
|
|
Serous |
68 (95%) |
|
Mucinous |
1 (1%) |
|
Endometroid |
2 (3%) |
|
Clear cell |
1 (1%) |
|
Baseline stage |
|
|
I |
7 (10%) |
|
II |
4 (6%) |
|
III |
48 (68%) |
|
IV |
13 (16%) |
|
Newly diagnosed cases |
5 (5%) |
|
Relapsed cases |
95 (95%) |
|
Family history |
|
|
Yes |
34 (34%) |
|
No |
38 (66%) |
|
Family history of breast cancer |
09 (26%) |
|
Ovarian cancer |
14 (41%) |
|
Both breast and ovarian cancer |
10 (29%) |
|
Prostate cancer |
01 (4%) |
|
Pathogenic/likely pathogenic (P/LP) |
32 (44%) |
|---|---|
|
Variant of unknown significance (VUS) |
10 (14%) |
|
Both P/LP and VUS |
04 (6%) |
|
Locus of P/LP mutations (n = 32) |
n (percentage) |
|
BRCA 1 |
24 (75%) |
|
BRCA 2 |
03 (10%) |
|
MER11 |
01 (3%) |
|
PALB2 |
01 (3%) |
|
RAD51 |
02 (6%) |
|
HMMR |
01 (3%) |
|
Patients characteristics |
Positive for P/LP mutation (n = 32) |
Negative for P/LP mutation (n = 40) |
p-Value |
|---|---|---|---|
|
Age |
47.5 ± 9 |
49.5 ± 12 |
0.77 |
|
Age group |
|||
|
< 50 years |
22 (68%) |
24 (60%) |
0.65 |
|
> 50 years |
10 (32%) |
16 (40%) |
|
|
Family history present (first or second-degree having breast/ovarian cancer) |
25 (78%) |
09 (22.5%) |
0.001 |
|
First-degree relatives |
24 (70%) |
5 (15%) |
|
|
Second-degree relatives |
5 (15%) |
02 (6%) |
|
|
Personal history of breast cancer |
05 (15%) |
03 (8%) |
0.31 |
|
NACT received |
17 (48%) |
19 (53%) |
0.20 |
|
Response to NACT |
|||
|
CR |
03 (18%) |
0 |
|
|
PR |
13 (76%) |
16 (84%) |
|
|
SD |
01 (06%) |
02 (10%) |
|
|
PD |
0 |
01 (05%) |
|
|
DFS (median) |
20.2 months |
21.4 months |
0.26 |
|
OS (median) |
91.4 months |
71.5 months |
0.38 |
Gene |
Site |
Variant |
Protein change |
Type of mutation |
|---|---|---|---|---|
|
BRCA 1 |
EXON 10 |
c.3607C > T |
p.Arg1203 Ter |
Nonsense |
|
BRCA 1 |
EXON 10 |
c.1450G > T |
p.Gly484 Ter |
Nonsense |
|
BRCA 1 |
EXON 10 |
c.1008delA |
p.Glu337LysfsTer4 |
Frameshift |
|
BRCA 1 |
EXON 10 |
c.3147delC |
p.Ser1050ValfsTer12 |
Frameshift |
|
BRCA 1 |
EXON 10 |
c.2769del |
p.Asn924IlefsTer76 |
Frameshift |
|
BRCA 1 |
EXON 10 |
c.2338C > T |
p.Gln780Ter |
Nonsense |
|
BRCA 1 |
EXON 10 |
c.2076_2080delTGACA |
p.His692GlnfsTer18 |
Frameshift |
|
BRCA 1 |
EXON 10 |
c.3607C > T |
p.Arg1203 Ter |
Nonsense |
|
BRCA 1 |
EXON 10 |
c.1953_1956delGAAA |
p.Lys653SerfsTer47 |
Frameshift |
|
BRCA 1 |
EXON 11 |
c.4183 C > T |
p.Gln1395Ter |
Nonsense |
|
BRCA 1 |
EXON 15 |
c.4738G > G/C |
p.Glu1580 Gln |
Missense |
|
BRCA 1 |
EXON 15 |
c.4571 C > A |
p.Ser1524Ter* |
Nonsense |
|
BRCA 1 |
EXON 15 |
c.4571 C > A |
p.Ser1524Ter* |
Nonsense |
|
BRCA 1 |
EXON 15 |
c.4571 C > A |
p.Ser1524Ter* |
Nonsense |
|
BRCA 1 |
EXON 15 |
c.4571 C > A |
p.Ser1524Ter* |
Nonsense |
|
BRCA 1 |
EXON 16 |
c.4900_4901delinsGCC |
p.Ser1634AlafsTer9 |
Frameshift |
|
BRCA 1 |
EXON 2 |
c.68_69 delAG |
p.Glu23ValfsTer17 |
Frameshift |
|
BRCA 1 |
EXON 2 |
c.68_69 delAG |
p.Glu23ValfsTer17 |
Frameshift |
|
BRCA 1 |
EXON 7 |
c.470_471 delCT |
p.Ser157Ter |
Nonsense |
|
BRCA 1 |
EXON 24 |
c.5572 T > C |
p.Trp1858Arg |
Missense |
|
BRCA 1 |
EXON 24 |
c.5572 T > C |
p.Trp1858Arg |
Missense |
|
BRCA 1 |
INTRON 17 |
c.5137 +1 G > A |
Splice site |
|
|
BRCA |
INTRON |
c.4547 + 1G > A |
Splice site |
|
|
1 |
14 |
|||
|
BRCA 1 |
LGR, EXON 1- 20 |
|||
|
BRCA 2 |
EXON 11 |
c.3182del |
p.Lys1061SerfsTer16 |
Frameshift |
|
BRCA 2 |
EXON 11 |
c.4570_4573delTTTC |
p.Phe1524IlefsTer18 |
Frameshift |
|
BRCA 2 |
EXON 11 |
c.5967 _5968 del |
p.Asp1990cysfsTer12 |
Frameshift |
|
RAD51 D |
EXON 5 |
c.423delA |
pAla142GlnfsTer14 |
Frameshift |
|
RAD51 D |
EXON 5 |
c.423delA |
pAla142GlnfsTer14 |
Frameshift |
|
HMMR |
EXON 12 |
c.1327C > T |
p.Gln443Ter |
Nonsense |
|
MER 11 |
EXON 10 |
c.1086del |
p.Val363TyrfsTer27 |
Frameshift |
|
PALB2 |
Exon 5 |
c.2488delG |
p.Glu830SerfsTer21 |
Frameshift |
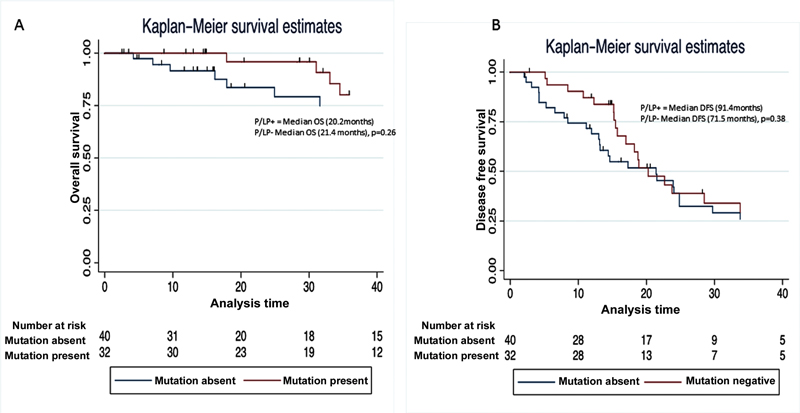
| Figure 1:Comparison of the (A) overall survival and (B) disease-free survival (DFS) of patients with and without a pathogenic/likely pathogenic (P/LP) mutation in any of the predisposing genes.
Cascade testing was advised for the family members of patients who were positive for the mutation. However, only 13 members from 8 different families underwent testing. Four were BRCA1 positive. Though risk reducing salpingo-oophorectomy was advised to the previvors, none of them had undergone the preventive surgery. All are under radiological surveillance.
Discussion
The current study is a cross-sectional analysis of selectively referred OC patients. We report 44% PLP mutations in this selected cohort of patients with carcinoma ovaries. BRCA mutations constituted 85% of all the mutations, while 15% of mutations were in non-BRCA genes. The prevalence of Ashkenazi Jewish founder mutation was very scarce (2.1%) in our cohort. The presence of BRCA mutation had no impact on survival outcomes. There was poor uptake of cascade testing.
In one retrospective analysis of OC (n = 238), where patients across India were selectively referred to the laboratory for multigene panel testing, 36% had pathogenic mutations (84.9% in BRCA1/2 gene and 15.1% in non-BRCA gene).[11] In another study of selectively referred patients with OC by Mehta et al from north India (n = 74), where only BRCA mutation testing was done, 41.5% of patients carried a mutation.[12] Both the above-mentioned studies with selective populations showed comparable mutation rates to our study. However, Gupta et al, in a prospective study (n = 239) of unselected patients of carcinoma ovary from all across India, reported BRCA mutations in 25.5% of patients, which may be closer to true prevalence in the population.[13]
Reported literature from various ethnicity worldwide has shown a slightly lower prevalence of BRCA and non-BRCA mutations. In a prospective study of consecutive patients of carcinoma ovary done by Eoh et al in Korea (n = 117), 32.5% of patients with epithelial ovarian cancer had a pathogenic mutation in BRCA and non-BRCA genes (79.5% mutation in BRCA gene).[14] In the Japanese nationwide multicentric study (n = 634), where OC patients were recruited prospectively, 28.5% of high-grade serous ovarian cancers had germline BRCA mutations.[15] Similarly, a retrospective study by Ataseven et al in the German population (n = 545) showed 29.5% P/LP mutations in hereditary cancer-predisposing genes. Eighty-one percent of mutations were in the BRCA gene alone.[16] Walsh et al showed that in the American population of prospectively selected OC (n = 360), 24% had germline mutation,18% in BRCA1/2, and 6% in non-BRCA genes.[5] In the study done at Royal Marsden Hospital, London, where only BRCA1 and BRCA2 were tested, the yield was 16%.[17]
The Ashkenazi Jewish founder mutation was present in two patients in our study. In another study done in North India, this mutation was found in only one patient.[12] However, in the study by Singh et al, which had patients from across India, this mutation was repeated 34 times.[11] In another study done exclusively in the South Indian population, which included both breast and OC, the Ashkenazi Jewish founder mutation was present in 10 out of 44 patients.[18] The profile of BRCA1/2 mutation in South India appears to be different from North India.
Patients with pathogenic mutations had a better response to platinum-based therapy. Eighteen percent of patients with mutations achieved CR postneoadjuvant chemotherapy compared with none in patients with no detectable mutations. This may be explained due to increased platinum sensitivity in BRCA deficient tumors. In BRCA deficient tumors xenograft studies have shown differential gene expression and pathway modulation, which includes upregulation of RAD 52 and ERCC1/RRM1 downregulation that that might be responsible for increased platinum sensitivity.[19] However, we did not find any difference in the PFS or OS. Some studies have reported longer PFS in BRCA mutated patients,[20] while others have shown no difference.[21] The heterogeneity in the outcome can be explained by other prognostic factors such as stage, optimal cytoreduction, and age.
Our study included multigene panel testing with reflex MLPA, and results were correlated with clinical details. This study reports the details exclusively from the North Indian population, chiefly Delhi and adjoining states, and the real-world challenges of access to testing, cascade testing, and counseling. Our study is the first study that uses mainstreaming of the genetic testing of OC by clinicians in India. Although no founder mutations were seen, there appears to be a significant difference in North-South Indian populations concerning the prevalence of Ashkenazi Jewish mutations.
Limitations
The patients in the study represent a highly selected population. It included those who were referred by the clinicians and who could avail of the testing. This selection may have its own biases. All patients could not undergo multigene panel testing, and some underwent BRCA only testing.Future Directions
This study and other contemporary studies point to the significant burden of a germline mutation in carcinoma of the ovary in the Indian population. The oncology community in India has accepted universal genetic testing for BRCA1/2 genes, and given the scarcity of counselors, this is imperative that all oncologists take part in mainstreaming the test. Locus-specific database specific to the Indian population is the need of the hour.
Conclusion
OC is an aggressive disease, and it has got high genetic predisposition. Knowing the deleterious mutations in various genes can help the patients and their biological relatives. This study reports various pathogenic and VUS mutations in BRCA and non-BRCA genes in the North Indian population. Forty-four percent of P/LP mutations were found with a very low frequency of founder mutations (2%). This study would help in building up the database for the Indian population.
Conflict of Interest
None declared.
Ethical Approval
The study protocol was approved by the Institute Ethics Committee vide letter number-IEC-511/5.6.20 RP/50/2020
Consent to Participate
Informed consent was obtained from all patients.
Availability of Data and Material
Data regarding this study will be available from the corresponding author (RP) at reasonable request.
Authors' Contributions
R.P; A.U; S.K; L.K; P.M; M.R; S.D; and S.K contributed to the concept design, patient referrals, and conduct of the study. R.G; D.T; and V.L.R were involved in laboratory testing. A.U; R.P did the statistical analysis.
Supplementary Material
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Stat 2020: report from national cancer registry programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Lin JJ, Egorova N, Franco R, Prasad-Hayes M, Bickell NA. Ovarian cancer treatment and survival trends among women older than 65 years of age in the United States, 1995-2008. Obstet Gynecol 2016; 127 (01) 81-89
- Torre LA, Trabert B, DeSantis CE. et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68 (04) 284-296
- Walsh T, Casadei S, Lee MK. et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 2011; 108 (44) 18032-18037
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007; 25 (11) 1329-1333
- Rebbeck TR, Lynch HT, Neuhausen SL. et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346 (21) 1616-1622
- Friedlander M, Moore KN, Colombo N. et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol 2021; 22 (05) 632-642
- Pujade-Lauraine E, Ledermann JA, Selle F. et al; SOLO2/ENGOT-Ov21 investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18 (09) 1274-1284
- Richards S, Aziz N, Bale S. et al; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17 (05) 405-424
- Singh J, Thota N, Singh S. et al. Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 2018; 170 (01) 189-196
- Mehta A, Vasudevan S, Sharma SK. et al. Germline BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance associated with breast/ovarian cancer: a report from North India. Cancer Manag Res 2018; 10: 6505-6516
- Gupta S, Rajappa S, Advani S. et al. Prevalence of BRCA1 and BRCA2 mutations among patients with ovarian, primary peritoneal, and fallopian tube cancer in India: a multicenter cross-sectional study. JCO Glob Oncol 2021; Jun;7: 849-861
- Eoh KJ, Kim J, Park HS. et al. Detection of germline mutations in patients with epithelial ovarian cancer using multi-gene panels: beyond BRCA1/2. Cancer Res Treat 2017; •••: 50
- Enomoto T, Aoki D, Hattori K. et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectional approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int J Gynecol Cancer 2019; 29 (06) 1043-1049
- Ataseven B, Tripon D, Rhiem K. et al. Prevalence of BRCA1 and BRCA2 mutations in patients with primary ovarian cancer - does the German checklist for detecting the risk of hereditary breast and ovarian cancer adequately depict the need for consultation?. Geburtshilfe Frauenheilkd 2020; 80 (09) 932-940
- Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients | Scientific Reports [Internet]. [cited 2020 Dec 28]. Accessed March 10, 2022 from: https://www.nature.com/articles/srep29506
- Rajkumar T, Meenakumari B, Mani S, Sridevi V, Sundersingh S. Targeted resequencing of 30 genes improves the detection of deleterious mutations in South Indian women with breast and/or ovarian cancers. Asian Pac J Cancer Prev 2015; 16 (13) 5211-5217
- Tassone P, Di Martino MT, Ventura M. et al. Loss of BRCA1 function increases the antitumor activity of cisplatin against human breast cancer xenografts in vivo. Cancer Biol Ther 2009; 8 (07) 648-653
- Tewari KS, Burger RA, Enserro D. et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 2019; 37 (26) 2317-2328
- Kotsopoulos J, Rosen B, Fan I. et al. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol 2016; 140 (01) 42-47
Address for correspondence
Raja Pramanik, MD, DMDepartment of Medical Oncology, Dr. B. R.A. Institute Rotary Cancer Hospital, All India Institute of Medical SciencesNew Delhi-110 029IndiaEmail: drrajapramanik@gmail.comPublication History
Article published online:
01 September 2022© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Comparison of the (A) overall survival and (B) disease-free survival (DFS) of patients with and without a pathogenic/likely pathogenic (P/LP) mutation in any of the predisposing genes.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Stat 2020: report from national cancer registry programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Lin JJ, Egorova N, Franco R, Prasad-Hayes M, Bickell NA. Ovarian cancer treatment and survival trends among women older than 65 years of age in the United States, 1995-2008. Obstet Gynecol 2016; 127 (01) 81-89
- Torre LA, Trabert B, DeSantis CE. et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68 (04) 284-296
- Walsh T, Casadei S, Lee MK. et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 2011; 108 (44) 18032-18037
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007; 25 (11) 1329-1333
- Rebbeck TR, Lynch HT, Neuhausen SL. et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346 (21) 1616-1622
- Friedlander M, Moore KN, Colombo N. et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol 2021; 22 (05) 632-642
- Pujade-Lauraine E, Ledermann JA, Selle F. et al; SOLO2/ENGOT-Ov21 investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18 (09) 1274-1284
- Richards S, Aziz N, Bale S. et al; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17 (05) 405-424
- Singh J, Thota N, Singh S. et al. Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 2018; 170 (01) 189-196
- Mehta A, Vasudevan S, Sharma SK. et al. Germline BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance associated with breast/ovarian cancer: a report from North India. Cancer Manag Res 2018; 10: 6505-6516
- Gupta S, Rajappa S, Advani S. et al. Prevalence of BRCA1 and BRCA2 mutations among patients with ovarian, primary peritoneal, and fallopian tube cancer in India: a multicenter cross-sectional study. JCO Glob Oncol 2021; Jun;7: 849-861
- Eoh KJ, Kim J, Park HS. et al. Detection of germline mutations in patients with epithelial ovarian cancer using multi-gene panels: beyond BRCA1/2. Cancer Res Treat 2017; •••: 50
- Enomoto T, Aoki D, Hattori K. et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectional approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int J Gynecol Cancer 2019; 29 (06) 1043-1049
- Ataseven B, Tripon D, Rhiem K. et al. Prevalence of BRCA1 and BRCA2 mutations in patients with primary ovarian cancer - does the German checklist for detecting the risk of hereditary breast and ovarian cancer adequately depict the need for consultation?. Geburtshilfe Frauenheilkd 2020; 80 (09) 932-940
- Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients | Scientific Reports [Internet]. [cited 2020 Dec 28]. Accessed March 10, 2022 from: https://www.nature.com/articles/srep29506
- Rajkumar T, Meenakumari B, Mani S, Sridevi V, Sundersingh S. Targeted resequencing of 30 genes improves the detection of deleterious mutations in South Indian women with breast and/or ovarian cancers. Asian Pac J Cancer Prev 2015; 16 (13) 5211-5217
- Tassone P, Di Martino MT, Ventura M. et al. Loss of BRCA1 function increases the antitumor activity of cisplatin against human breast cancer xenografts in vivo. Cancer Biol Ther 2009; 8 (07) 648-653
- Tewari KS, Burger RA, Enserro D. et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 2019; 37 (26) 2317-2328
- Kotsopoulos J, Rosen B, Fan I. et al. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol 2016; 140 (01) 42-47


 PDF
PDF  Views
Views  Share
Share

