Current Treatment Options for Human Epidermal Growth Factor Receptor 2-Directed Therapy in Metastatic Breast Cancer: An Indian Perspective
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(03): 368-379
DOI: DOI: 10.4103/ijmpo.ijmpo_201_17
Abstract
Human epidermal growth factor receptor 2 (HER2)-positive is an aggressive subtype of breast cancer and has historically been associated with poor outcomes. The availability of various anti-HER2 therapies, including trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (TDM-1), has remarkably improved the clinical outcomes in patients with HER2-positive metastatic breast cancer (mBC). However, there is a need to optimize treatment within this population, given the wide variability in clinical presentation. Additionally, geographical and socio-economic considerations too need to be taken into account. To clarify and collate evidence pertaining to HER2-positive metastatic breast cancer, a panel of medical and clinical oncologists from across India developed representative clinical scenarios commonly encountered in clinical practice in the country. This was followed by two meetings wherein each clinical scenario was discussed in detail and relevant evidence appraised. The result of this process is presented in this manuscript as evidence followed by therapeutic recommendations of this panel for management of HER2-positive mBC in the Indian population.
Keywords
Human epidermal growth factor receptor 2 positive - India - metastatic breast cancer - targeted therapyPublication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Human epidermal growth factor receptor 2 (HER2)-positive is an aggressive subtype of breast cancer and has historically been associated with poor outcomes. The availability of various anti-HER2 therapies, including trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (TDM-1), has remarkably improved the clinical outcomes in patients with HER2-positive metastatic breast cancer (mBC). However, there is a need to optimize treatment within this population, given the wide variability in clinical presentation. Additionally, geographical and socio-economic considerations too need to be taken into account. To clarify and collate evidence pertaining to HER2-positive metastatic breast cancer, a panel of medical and clinical oncologists from across India developed representative clinical scenarios commonly encountered in clinical practice in the country. This was followed by two meetings wherein each clinical scenario was discussed in detail and relevant evidence appraised. The result of this process is presented in this manuscript as evidence followed by therapeutic recommendations of this panel for management of HER2-positive mBC in the Indian population.
Keywords
Human epidermal growth factor receptor 2 positive - India - metastatic breast cancer - targeted therapyIntroduction
An estimated 15%–20% of women with breast cancer overexpress human epidermal growth factor receptor 2 (HER2).[1],[2] Ghosh et al. reported that 16.7% and 8.1% of patients (n = 2001) with breast cancer presenting at their referral cancer center in Mumbai had HER2 immunochemistry (IHC) scores of 3+ and 2+, respectively,[3] while Doval et al. found that 23% of a cohort of 1284 breast cancer patients from their institution in New Delhi were HER2 positive.[4] More recently, Chatterjee et al.[5] and Agrawal et al.[6] have reported HER2-positive rates in locally advanced breast cancers to be as high as 38%–40 % based on the use of quality-assured automated IHC and fluorescence in situ hybridization tests.
HER2-positive subtype of breast cancer has historically been associated with poor clinical outcomes.[7] The disease course is typically aggressive with a high propensity for early metastases, relapse, and shorter survival than other subtypes. In this backdrop, the use of several HER2-directed therapies [Table 1] has substantially improved the outcomes in this population.[8],[9],[10],[11] These anti-HER2 therapies are available in India.
|
Anti-HER2 agent |
Indication |
Year of approval by the US FDA |
|---|---|---|
|
FDA – Food and Drug Administration; HER2 – Human epidermal growth factor receptor 2; mBC – Metastatic breast cancer; T-DM1 – Trastuzumab emtansine |
||
|
Trastuzumab |
Trastuzumab (Herceptin™) combined with paclitaxel in patients with mBC whose tumors overexpress HER2 protein and who have not received chemotherapy for their metastatic disease |
1998 |
|
Lapatinib |
Lapatinib (Tykerb®) for use in combination with capecitabine for treatment of patients with advanced breast cancer or mBC whose tumors overexpress HER2 (ErbB2), and who have received prior therapy including anthracycline, taxane, and trastuzumab |
2007 |
|
Pertuzumab |
Pertuzumab (Perjeta™) for use in combination with trastuzumab and docetaxel for the treatment of HER2-positive mBC who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease |
2012 |
|
T-DM1 |
Trastuzumab |
|
|
emtansine (Kadcyla™) for use as a single agent for the treatment of patients with HER2-positive mBC, who had previously received treatment with trastuzumab and taxane, either separately or in combination |
2013 |
|
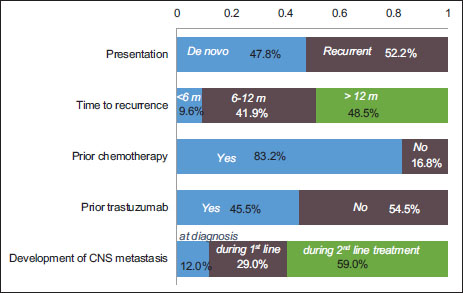
| Figure 1:Profile of patients with human epidermal growth factor receptor 2‑positive metastatic breast cancer, based on a survey of expert panel of nine medical oncologists across India. (Note: The graph represents responses received from the experts, and the total may not exactly equal 100%). HER2 – Human epidermal growth factor receptor 2
The panel discussed the choice of treatment for each clinical scenario based on the available evidence from relevant Phase 3 randomized controlled trials. The final treatment recommendation was reached by consensus or vote. Panelists were asked to indicate their choices on the assumption that the patients had access to all approved anti-HER2 drugs and were able to afford treatment or were adequately covered by insurance.
Clinical Scenario 1: first-Line Treatment for Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer
A 56-year-old woman with HER2-positive, hormone receptor-negative breast cancer underwent surgery for a 25-mm, Grade 3 invasive duct cancer. Postoperatively, she was started on doxorubicin and cyclophosphamide, followed by paclitaxel and trastuzumab (ACσ TH regimen), followed by continuation of adjuvant trastuzumab, for a total anti-HER2 therapy duration of 1 year. Two years after completion of adjuvant trastuzumab therapy, she developed biopsy-confirmed HER2-positive, hormone receptor-negative metastatic disease in the liver.
In addition, two subscenarios in the first-line metastatic breast cancer (mBC) setting were also considered. In the first subscenario, the same representative patient as above had received a similar treatment with the exception that she did not receive adjuvant trastuzumab (i.e., she was trastuzumab naïve at the time of developing metastases). In the second subscenario, the same patient as above presents with de novo HER2-positive mBC at the time of initial diagnosis.
The following therapeutic options were discussed for this scenario and subscenarios:
-
Combination of trastuzumab and taxane
-
Combination of lapatinib and taxane
-
Combination of pertuzumab, trastuzumab, and docetaxel
-
Combination of trastuzumab, paclitaxel, and everolimus
-
Trastuzumab emtansine (T-DM1).
Literature analysis
The Herceptin trial (H0648 g) conducted by Slamon et al.[8] was the first large Phase 3 trial to demonstrate that targeting a specific dysfunctional genetic alteration in a human solid tumor was feasible and could lead to clinical benefits. This pivotal study included 450 women with HER2-positive mBC who had not previously received chemotherapy for metastatic disease, but could have received anthracycline in the (neo) adjuvant setting (were treated with trastuzumab and paclitaxel).[12] Clinical outcomes in the trastuzumab plus chemotherapy arm were significantly superior to those in the chemotherapy-alone arm [Table 2], although concurrent anthracycline and trastuzumab had increased the risk of cardiac dysfunction and led to the approval of Herceptin with paclitaxel in HER2-positive mBC by the US-Food and Drug Administration. The MA 31 trial was an interesting comparison between different approaches to HER2 blockade in mBC – oral small molecule versus intravenous antibody. It compared the efficacy of lapatinib versus trastuzumab in combination with taxanes as first-line treatment for HER2-positive mBC. After 24 weeks of chemotherapy plus HER2-directed therapy, the respective anti-HER2 monotherapy was continued until disease progression. In both arms, 18% patients had received prior (neo) adjuvant trastuzumab therapy and 42% of patients had de novo mBC at primary diagnosis. The results showed that patients in the trastuzumab arm had longer progression-free survival (PFS) than those in the lapatinib arm [Table 2]; however, there was no significant difference in overall survival (OS) between the two arms.[13] Patients treated with lapatinib displayed characteristic adverse effects, especially gastrointestinal and skin toxicity.
|
Trial |
Treatment arms |
Treatment-specific criteria |
Results |
|---|---|---|---|
|
*Chemotherapy consisted of an anthracycline plus cyclophosphamide for patients who had never before received anthracycline or paclitaxel for patients who had received adjuvant (postoperative) anthracycline. HER2+ – Human epidermal growth factor receptor 2 positive; LABC – Locally advanced breast cancer; MBC – Metastatic breast cancer; OS – Overall survival; PFS – Progression-free survival; TTP – Time to progression; T-DM1 – Trastuzumab emtansine; RR – Relative risk; HR – Hazard ratio; NA – Not available; BC – Breast Cancer |
|||
|
H0648g[8] |
Chemotherapy* + trastuzumab (n=235) versus chemotherapy (n=234) |
HER2-positive mBC; no previous chemotherapy for metastatic disease |
Trastuzumab + chemotherapy versus chemotherapy Median TTP: 7.4 months versus 4.6 months (RR: 0.51; P<0 P=0.046) class="i">n=92) versus paclitaxel (n=96) Median TTP: 6.9 months versus 3.0 months (RR: 0.38; P<0 P=0.17)> |
|
MA.31[13] |
Lapatinib + taxane (n=326) versus trastuzumab + taxane (n=326) |
HER2-positive mBC; prior (neo) adjuvant trastuzumab and/or taxane therapy allowed provided last dose ≥1 year before randomization (18% patients) |
Lapatinib + taxane versus trastuzumab + taxane Median PFS: 9 months versus 11.3 months (HR: 1.37; P=0.001) Median OS: Median not observed (HR: 1.28; P=0.11) |
|
Trastuzumab + docetaxel + pertuzumab (n=402) versus trastuzumab + docetaxel + placebo (n=406) |
Locally recurrent, unresectable, or metastatic HER2+ve BC; Prior (neo) adjuvant chemotherapy including trastuzumab and/or taxane allowed if ≥1 year has elapsed between treatment completion and detection of mBC |
Trastuzumab + docetaxel + pertuzumab versus trastuzumab + docetaxel + placebo Overall population Median PFS: 18.5 months versus 12.4 months (HR: 0.62; P<0 class="i">neo) adjuvant trastuzumab-treated patients (12%, n=88) Median PFS: 16.9 months versus 10.4 months (HR: 0.62) Median OS: 53.8 months versus 46.2 months (HR: 0.68) |
|
|
BOLERO-1[16] |
Trastuzumab + paclitaxel + everolimus (n=480) versus trastuzumab + paclitaxel + placebo (n=239) |
Locally recurrent, unresectable, or metastatic HER2+ve BC; no prior anthracycline/taxane in the metastatic setting; previous (neo) adjuvant trastuzumab and chemotherapy allowed, if >12 months elapsed at the date of randomization (11% patients) |
Trastuzumab + everolimus versus trastuzumab + placebo Median PFS: 14.95 months versus: 14.49 months (HR: 0.89; P=0.1166) |
|
MARIANNE1171 |
T-DM1 (n=367) versus T-DM1 + pertuzumab (n=363) versus trastuzumab + taxane (n=365) |
Unresectable- progressive- or recurrent locally advanced, or previouslyuntreated MBC; prior (neo) adjuvant chemotherapy with vinca alkaloid or taxane allowed if >6 months since diagnosis of advanced BC |
T-DM1 versus trastuzumab + taxane Overall patient population Median PFS: 14.1 months versus 13.7 months (HR: 0.91; P=0.31) Prior (neo) adjuvant trastuzumab-/lapatinib-treated patients (31%, n=226) Median PFS: 15.2 months versus 10.3 months (HR: 0.75) T-DM1 + pertuzumab versus trastuzumab + taxane Overall population Median PFS: 15.2 months versus 13.7 months (HR: 0.87; P=0.14) Median OS was not reached in any treatment group |
|
T-DM1 (n=495) versus lapatinib + capecitabine (n=496) |
Unresectable locally advanced or metastatic HER2+ve BC: Prior taxane and trastuzumab; progression during treatment for metastasis, or progression within 6 months of adjuvant treatment (early relapse |
T-DM1versus lapatinib plus T-DM1versus lapatinib plus capecitabine Overall population Median PFS: 9.6 months versus 6.4 months (HR: 0.65; P<0 n=118)> |
|
|
Trials |
Treatment arms |
Treatment‑specific criteria |
Results |
|---|---|---|---|
|
HER2 – Human epidermal growth factor receptor 2; LABC – Locally advanced breast cancer; mBC – Metastatic breast cancer; OS – Overall survival; PFS – Progression‑free survival; T‑DM1 – Trastuzumab emtansine; TTP – Time to progression; HR – Hazard ratio; NR=Statistical significance for OS cannot be claimed because of the hierarchical testing of OS after the primary PFS end point |
|||
|
Capecitabine + lapatinib (n=198) versus capecitabine (n=201) |
HER2-positive LABC or mBC that progressed after prior treatment with anthracycline, taxane, and trastuzumab |
Capecitabine + lapatinib versus capecitabineMedian TTP: 6.2 months versus 4.3 months (HR: 0.57; P=<0 P=0.210)> |
|
|
EMILIA[11] |
T-DM1 (n=495) versus lapatinib + capecitabine (n=496) |
Unresectable locally advanced or metastatic HER2+ve BC: Prior taxane and trastuzumab; progression during treatment for metastasis, or progression within 6 months of adjuvant treatment |
T-DM1 versus lapatinib + capecitabineMedian PFS: 9.6 months versus 6.4 months (HR: 0.65; P<0> |
|
BOLERO-3[30] |
Trastuzumab + vinorelbine + everolimus (n=284) versus trastuzumab + vinorelbine + placebo (n=285) |
LABC or metastatic HER2-positive breast cancer: Recurrence during or <12> |
Trastuzumab + vinorelbine + everolimus versus trastuzumab + vinorelbineMedian PFS: 7 months versus 5.8 months (HR: 0.78; P=0.0067) |
|
LUX breast-1[31] |
Vinorelbine + afatinib (n=339) versus vinorelbine + trastuzumab (n=169) |
HER2-positive mBC who had progressed on or <12> |
Vinorelbine + afatinib versus vinorelbine + trastuzumab Median PFS: 5.5 months versus months (HR: 1.10; P=0.043) Median OS: 20.5 months versus 28.6 months (HR: 1.48; P=0.048) |
|
Pertuzumab + trastuzumab + capecitabine (n=228) versus trastuzumab + capecitabine (n=224) |
HER2-positive mBC: Experienced disease progression during or after trastuzumab-based therapy; received a prior taxane |
Pertuzumab + trastuzumab + capecitabine versus trastuzumab + capecitabineMedian PFS 11.1 months versus 9 months (HR: 0.82; P=0.0731) Median OS 36.1 months versus 28.1 months (HR: 0.68; P=NR) |
|
The BOLERO 3 study was conducted to assess whether the addition of the mTOR inhibitor everolimus to trastuzumab would restore sensitivity to trastuzumab. In patients with HER2-positive, trastuzumab-resistant advanced breast carcinoma, there was a significant improvement in the median PFS in favor of everolimus versus the placebo group [Table 3]; however, 42% of patients in the everolimus arm experienced a serious adverse event.[30]
In the LUX Breast-1 study, patients who progressed on trastuzumab fared better on further trastuzumab than on the pan-HER2 inhibitor afatinib [Table 3]. The reason could be related to the differing mechanisms of antibody versus small molecule, as well as to the immunological effects. However, the treatment was associated with an unfavorable benefit/risk ratio.[31]
The results of PHEREXA trial presented at the ASCO 2016 showed that addition of pertuzumab to trastuzumab plus capecitabine as the second-line treatment of patients with mBC did not significantly improve PFS.[32],[33]
Panel recommendation
On a review of all trials in the context of patients who progressed on trastuzumab, a survival advantage was evident only in the EMILIA trial in those treated with T-DM1. In the absence of cost constraints, T-DM1 would be the preferred choice in this setting.
The panel also recommends that in patients with HER2-positive mBC who show indolent progression on a trastuzumab-based regimen, a change of the chemotherapy partner or re-introduction of chemotherapy, with continuation of trastuzumab, could also be a viable option.
Clinical Scenario 3: Central Nervous System Metastases in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer
A 52-year-old woman presented with HER2-positive, hormone receptor-positive, invasive ductal carcinoma of the breast (T2N1M0; 32 mm, Grade 3 with 5 of 14 lymph nodes involved). She received adjuvant chemotherapy with docetaxel in combination with carboplatin and trastuzumab (TCH) and continued on trastuzumab monotherapy (to complete 1 year) with aromatase inhibitor. One year later, she developed biopsy-confirmed HER2-positive and hormone receptor-positive metastases in liver. She received six cycles of paclitaxel with trastuzumab. After 6 months on trastuzumab monotherapy, she developed multiple unresectable central nervous system (CNS) metastasis.
For this scenario, the following treatment regimens were considered for the discussion:
-
Continue trastuzumab with locoregional treatment of CNS metastasis
-
Change to a combination of lapatinib and capecitabine
-
Locoregional treatment of CNS metastasis followed by T-DM1.
Literature analysis
There is some evidence, although not from randomized trials, that use of chemotherapy and HER2-targeted therapy in addition to brain radiation might improve OS in patients with CNS metastasis.[34]
Human epidermal growth factor receptor 2-positive metastatic breast cancer without central nervous system metastasis at baseline
A retrospective study by Park et al. showed that addition of trastuzumab to chemotherapy increased the time to onset of CNS metastasis (15 months vs. 10 months, P = 0.035) and time to death from CNS metastases (14.9 months vs. 4.0 months, P = 0.0005) in patients with HER2-positive mBC. However, among patients with HER2-positive disease, the incidence of CNS metastasis in those treated with trastuzumab (37.8%) was higher than that in patients who were not treated with trastuzumab (25.0%; P = 0.028).[35] It is worth noting that following radiation therapy, the concentration of trastuzumab in the cerebrospinal fluid tends to increase.[36]
In the CEREBEL trial, no significant difference was observed between the lapatinib plus capecitabine and the trastuzumab plus capecitabine arms with respect to time to metastasis and development of new CNS metastasis [5.7 and 4.4 months, respectively; [Table 4]. Further, there was low overall incidence of CNS progression at any time (7% and 6%, respectively).[37]
|
Trials |
Treatment arms |
Treatment-specific criteria |
Results |
|---|---|---|---|
|
@Exploratory analysis; *Post hoc analysis; $ ≥50%-volumetric reduction of CNS lesions in the absence of increased steroid use, progressive neurological symptoms and progressive extra-CNS disease; #Patients with asymptomatic CNS metastases previously treated with radiotherapy were eligible to enroll 14 days after last radiotherapy treatment. CNS – Central nervous system; HER2 – Human epidermal growth factor receptor 2; LABC – Locally advanced breast cancer; mBC – Metastatic breast cancer; MRI – Magnetic resonance imaging; HR – Hazard ratio; OS – Overall survival; PFS – Progression-free survival; T-DM1 – Trastuzumab emtansine; TTP – Time to progression |
|||
|
HER2-positive mBC without CNS metastasis at baseline |
|||
|
CEREBEL[37] |
Capecitabine + lapatinib (n=271) versus capecitabine + trastuzumab (n=269) |
HER2-positive mBC without CNS metastasis at baseline |
Capecitabine + lapatinib versus capecitabine + trastuzumab Incidence of CNS metastasis as first site of relapse: 3% versus 5% (HR: 0.65; P=0.36) Median PFS: 6.6 months versus 8.1 months; (HR: 1.30; P=0.021) Median OS: 22.7 months versus 27.3 months; (HR: 1.34; P=0.095) |
|
CLEOPATRA@[38] |
Trastuzumab + docetaxel + pertuzumab (n=55) versus trastuzumab + docetaxel + placebo (n=51) |
Patients without CNS metastasis at baseline |
Pertuzumab arm versus placebo arm Median TTP in CNS: 15 months versus 11.9 months (HR: 0.59; P=0.0049) Median OS in patients with CNS progression 34.4 months versus 26.3 months (HR: 0.66; P=0.1139) |
|
HER2-positive mBC with CNS metastasis at baseline |
|||
|
LANDSCAPE[39] |
Lapatinib + capecitabine (n=45) |
HER2-positive mBC: At least one measurable CNS lesion of ≥10 mm in diameter on MRI |
Objective CNS response$: 65.9% |
|
EMILIA*[40] |
T-DM1 (N=45) versus lapatinib + capecitabine (n=50) |
HER2-positive mBC patients who had stable CNS disease at baseline# |
T-DM1 versus lapatinib+capecitabine Median PFS: 5.9 months versus 5.7 months (HR: 1; P=1.000) Median OS: 26.8 m vs. 12.9 m (HR: 0.38; P=0.0081) |
|
LUX breast-3[41] |
Vinorelbine + afatinib (n=38) versus Afatinib (n=40) versus investigator’s choice |
HER2-positive breast cancer with documented CNS recurrence/ progression (on imaging) during or after trastuzumab and/or lapatinib-based therapy |
Patient benefit at 12 weeks (absence of CNS or extra‑CNS disease progression, no tumor-related worsening of neurological signs or symptoms, and no increase in corticosteroid dose) Vinorelbine + afatinib, 34·2 |
|
Trial |
Treatment arms |
Treatment-specific criteria |
Results |
|---|---|---|---|
|
HER2 – Human epidermal growth factor receptor 2; LABC – Locally advanced breast cancer; mBC – Metastatic breast cancer; MRI – Magnetic resonance imaging; HR – Hazard ratio; OS – Overall survival; PFS – Progression‑free survival; T-DM1 – Trastuzumab emtansine |
|||
|
T-DM1 (n=404) versus treatment of physician’s choice (n=198) |
HER2-positive advanced breast cancer: ≥2 prior HER2-directed therapies for advanced breast cancer; prior treatment with trastuzumab, lapatinib, taxane |
T-DM1 versus treatment of physician’s choice Median PFS: 6.2 months versus 3.3 months (HR: 0.53; P<0 P=0.0002)> |
|
|
Lapatinib (n=146) with trastuzumab versus lapatinib alone (n=145) |
HER2-positive mBC: Prior taxane, anthracycline, and trastuzumab; prior HER2-directed therapies for advanced breast cancer; Progression on trastuzumab within most recent regimen for mBC |
Lapatinib with trastuzumab versus lapatinib alone Median PFS: 12.0 weeks versus 8.1 weeks, (HR: 0.73; P=0.008) Median OS: 14 months versus 9.5 months, (HR: 0.74; P=0.026) |
|

| Figure 1:Profile of patients with human epidermal growth factor receptor 2‑positive metastatic breast cancer, based on a survey of expert panel of nine medical oncologists across India. (Note: The graph represents responses received from the experts, and the total may not exactly equal 100%). HER2 – Human epidermal growth factor receptor 2
References
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004; 5: 63-9
- Saini KS, Azim HAJr, Metzger-Filho O, Loi S, Sotiriou C, de Azambuja E. et al. Beyond trastuzumab: New treatment options for HER2-positive breast cancer. Breast 2011; 20 Suppl 3: S20-7
- Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P. et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer 2011; 48: 391-6
- Doval DC, Sharma A, Sinha R, Kumar K, Dewan AK, Chaturvedi H. et al. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in New Delhi, India. Asian Pac J Cancer Prev 2015; 16: 4959-64
- ed">5 Chatterjee S, Saha A, Arun I, Nayak SS, Sinha S, Agrawal S. et al. Correlation of clinicopathological outcomes with changes in IHC4 status after NACT in locally advanced breast cancers: Do pre-NACT ER/PR status act as better prognosticators?. Breast Cancer (Dove Med Press) 2015; 7: 381-8
- Agrawal S, Banswal L, Saha A, Arun I, Datta SS, Chatterjee S. et al. Progesterone receptors, pathological complete response and early outcome for locally advanced breast cancer – A single centre study (PPLB - 01). Indian J Surg Oncol 2016; 7: 397-406
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177-82
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783-92
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733-43
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH. et al Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012; 366: 109-19
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012; 367: 1783-91
- Genentech (A Member of the Roche group); Herceptin® (Trastuzumab) Development Timeline. Available from: https://www.gene.com/media/product-information/herceptin-development-timeline. [Last accessed on 2017 Jun 19].
- Gelmon KA, Boyle FM, Kaufman B, Huntsman DG, Manikhas A, Di Leo A. et al Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer: Final results of NCIC CTG MA. 31. J Clin Oncol 2015; 33: 1574-83
- Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372: 724-34
- Ciruelos Gil EM, Brufsky A, Im YH, Kim SB, Sinha S, Knott A. et al Efficacy and safety of first-line (1L) pertuzumab (P), trastuzumab (T), and docetaxel (D) in HER2-positive MBC (CLEOPATRA) in patients previously exposed to trastuzumab. J Clin Oncol 2013; 31 15 Suppl: 600
- Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP. et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015; 16: 816-29
- Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P. et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: Primary results from the phase III MARIANNE study. J Clin Oncol 2017; 35: 141-8
- Kadycla. Summary of Product Characteristics. Available from: https://www.ec.europa.eu/health/documents/community-register/2013/20131115127009/anx_127009_en.pdf. [Last accessed on 2017 Jun 19].
- Swain SM, Clark E, Baselga J. Treatment of HER2-positive metastatic breast cancer. N Engl J Med 2015; 372: 1964-5
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381: 805-16
- Baselga J, Cortes J, Im SA, Pivot XB, Clark E, Knott A. et al. Adverse events with pertuzumab and trastuzumab: Evolution during treatment with and without docetaxel in CLEOPATRA. J Clin Oncol 2012; 30: 597
- Miles D, Puglisi F, Schneeweiss A, Ciruelos E, Peretz-Yablonski T, Moreno K. et al. 1816 preliminary safety results from PERUSE, a study of 1436 patients (pts) treated with first-line pertuzumab (P) combined with trastuzumab (H) and taxane therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC). Eur J Cancer 2015; 51: S
- Robert NJ, Goertz HP, Chopra P, Jiao X, Yoo B, Patt D. et al. HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: Treatment patterns and outcomes. Drugs Real World Outcomes 2017; 4: 1-7
- Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M. et al Targeting the PI3K/AKT/mTOR and raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 2013; 39: 935-46
- Yardley DA, Kaufman PA, Brufsky A, Yood MU, Rugo H, Mayer M. et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2014; 145: 725-34
- Wong NS, Anderson BO, Khoo KS, Ang PT, Yip CH, Lu YS. et al. Management of HER2-positive breast cancer in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009; 10: 1077-85
- Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL. et al. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2016; 34: 902-9
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008; 112: 533-43
- Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: Final survival analysis of a phase III randomized trial. Oncologist 2010; 15: 924-34
- André F, O'Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G. et al Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014; 15: 580-91
- Harbeck N, Huang CS, Hurvitz S, Yeh DC, Shao Z, Im SA. et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-breast 1): An open-label, randomised, phase 3 trial. Lancet Oncol 2016; 17: 357-66
- Urruticoechea A, Rizwanullah M, Im S, Sánchez-Ruiz AC, Lang I, Tomasello G. et al. PHEREXA: A phase III study of trastuzumab + capecitabine +/- pertuzumab for patients who progressed during/after one line of trastuzumab-based therapy in the HER2-positive metastatic breast cancer setting. J Clin Oncol 34 (Suppl. 15) 20.05.2016; Abstract 504
- Urruticoechea A, Rizwanullah M, Im SA, Ruiz ACS, Láng I, Tomasello G. et al Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J Clin Oncol 2017; 35: 3030-8
- Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim TM. et al. Clinical outcome of central nervous system metastases from breast cancer: Differences in survival depending on systemic treatment. J Neurooncol 2012; 106: 303-13
- Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH. et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 2009; 100: 894-900
- Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 2007; 18: 23-8
- Pivot X, Manikhas A, Żurawski B, Chmielowska E, Karaszewska B, Allerton R. et al CEREBEL (EGF111438): A Phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015; 33: 1564-73
- Swain SM, Baselga J, Miles D, Im YH, Quah C, Lee LF. et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann Oncol 2014; 25: 1116-21
- Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F. et al Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol 2013; 14: 64-71
- Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann Oncol 2015; 26: 113-9
- Cortés J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz Z. et al Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-breast 3): A randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 2015; 16: 1700-10
- Montemurro F, Ellis P, Delaloge S, Wuerstlein R, Anton A, Button P. et al. Safety and efficacy of trastuzumab emtansine (T-DM1) in 399 patients with central nervous system metastases: Exploratory subgroup analysis from the KAMILLA study. Cancer Res 2017; 77 4 Suppl: P1-12-10
- Krop IE, Kim SB, González-Martín A, LoRusso PM, Ferrero JM, Smitt M. et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 689-99
- Wildiers H, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Yu R. et al. Trastuzumab emtansine improves overall survival versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer: Final overall survival results from the phase 3 TH3RESA study. Cancer Res 2016; 76 (4 Suppl): S5-05
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M. et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with erbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010; 28: 1124-30
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G. et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol 2012; 30: 2585-92


 PDF
PDF  Views
Views  Share
Share

