Cyclin D1 expression in multiple myeloma by immunohistochemistry: Case series of 14 patients and literature review
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 283-291
DOI: DOI: 10.4103/0971-5851.125246
Abstract
Background: Cyclin D1 dysregulation is an early and unifying oncogenic event in patients of multiple myeloma (MM). This may be detected up to 30% cases by immunohistochemistry (IHC) and up to 40-50% cases by molecular studies. However, studies on the clinical significance of cyclin D1 dysregulation in MM have been inconclusive. We aimed to study the pattern of cyclin D1 expression in MM by IHC and correlate with selected clinicopathologic features. Materials and Methods: Formalin fixed, decalcified, bone marrow trephine sections from 14 symptomatic patients of MM (13 newly diagnosed and one relapsed) were subjected to cyclin D1 IHC by using a rabbit monoclonal antibody to cyclin D1 (clone EPR2241). Results: Cyclin D1 [removed]in ≥10% tumor cell nuclei) was observed in 8 of 14 cases (57%). Cyclin D1 positive (+) group had significantly lower hemoglobin level (P = 0.03) than cyclin D1 negative (−) group (n = 6); though both groups showed no statistical significance (P > 0.05) in regard to age, gender, Durie and Salmon stage, lytic bone lesions, light chain phenotype, creatinine, calcium, lactate dehydrogenase, leukocyte and platelet count and bone marrow histology. Ten of 14 (71.5%) showed a favorable response (follow-up; 7 days to 34 months) to thalidomide and/or bortezomib based chemotherapeutic regimen. Four of eight cyclin D1+ patients showed complete response, two had a partial response (PR) and two died of the disease; whereas 4/6 cyclin D1− patients had PR, one refused definitive therapy and one was lost to follow-up (P > 0.05, Fischer′s exact test). Conclusion: IHC may be a feasible tool for the demonstration of cyclin D1 expression on adequately processed trephine biopsy specimen in MM patients in a resource poor setting. Negative IHC results should be correlated with molecular techniques for prognostication.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Cyclin D1 dysregulation is an early and unifying oncogenic event in patients of multiple myeloma (MM). This may be detected up to 30% cases by immunohistochemistry (IHC) and up to 40-50% cases by molecular studies. However, studies on the clinical significance of cyclin D1 dysregulation in MM have been inconclusive. We aimed to study the pattern of cyclin D1 expression in MM by IHC and correlate with selected clinicopathologic features.
Materials and Methods:
Formalin fixed, decalcified, bone marrow trephine sections from 14 symptomatic patients of MM (13 newly diagnosed and one relapsed) were subjected to cyclin D1 IHC by using a rabbit monoclonal antibody to cyclin D1 (clone EPR2241).
Results:
Cyclin D1 [removed]in ≥10% tumor cell nuclei) was observed in 8 of 14 cases (57%). Cyclin D1 positive (+) group had significantly lower hemoglobin level (P = 0.03) than cyclin D1 negative (−) group (n = 6); though both groups showed no statistical significance (P > 0.05) in regard to age, gender, Durie and Salmon stage, lytic bone lesions, light chain phenotype, creatinine, calcium, lactate dehydrogenase, leukocyte and platelet count and bone marrow histology. Ten of 14 (71.5%) showed a favorable response (follow-up; 7 days to 34 months) to thalidomide and/or bortezomib based chemotherapeutic regimen. Four of eight cyclin D1− patients showed complete response, two had a partial response (PR) and two died of the disease; whereas 4/6 cyclin D1 − patients had PR, one refused definitive therapy and one was lost to follow-up (P > 0.05, Fischer's exact test).
Conclusion:
IHC may be a feasible tool for the demonstration of cyclin D1 expression on adequately processed trephine biopsy specimen in MM patients in a resource poor setting. Negative IHC results should be correlated with molecular techniques for prognostication.
INTRODUCTION
Multiple myeloma (MM) is a neoplastic plasma cell disorder that is characterized by clonal proliferation of malignant plasma cells in the bone marrow microenvironment, monoclonal protein in serum and/or urine and associated organ dysfunction. This accounts for approximately 1% of neoplastic diseases and 13% of all hematologic malignancies. The median age at diagnosis is approximately 70 years; 37% of patients are younger than 65 years, 26% are between the ages 65 and 74 years and 37% are 75 years of age or older.[1] The diagnosis is based upon the combination of clinicopathological, radiological and biochemical parameters.[1,2,3]
Cyclin D1 is a product of CCND1 gene which, in association with cyclin dependent kinase, causes phosphorylation of retinoblastoma gene product leading to release of the transcription factor E2F. This in turn, causes G1 to S phase transition allowing DNA replication and increased cellular proliferation. Overexpression of cyclin D1 by either translocation t (11;14) (q13;q32) (most common) or gene amplification is an early and unifying oncogenic event in several human cancers, most commonly mantle cell lymphoma and MM. Gene expression profiling and fluorescence in-situ hybridization (FISH) studies have identified prognostically significant and diverse genotypic variants of MM.[4,5,6,7] Essentially, all cases of myeloma are associated with dysregulation of cyclin D1, D2 or D3 expression, which may have prognostic significance. Cases with dysregulation of cyclin D1 or D3 have been associated with a favorable prognosis compared with cyclin D2 positive cases.[8] Although, most studies dealing with the prognostic significance of cyclin D1 in MM have been performed by using cell lines, microarrays or FISH techniques; recent studies have shown the utility of immunohistochemistry (IHC) in the prognostic evaluation in myeloma.[8,9,10,11,12,13]
The aim of the present study was to evaluate the immunohistochemical expression of cyclin D1 in a series of myeloma patients and correlate with clinicopathological features along with a brief review of relevant literature.
MATERIALS AND METHODS
We evaluated bone marrow aspirate and trephine biopsy specimen from 14 patients of MM (13 newly diagnosed and one at relapse) in the Department of Pathology of our Institute from January 2011 to September 2012. The Institutional Ethics Committee of our Institute approved the research study and in all, informed consent was obtained from the patients or their relatives in accordance with the Declaration of Helsinki. The diagnosis of MM was based upon a combination of pathological, radiological, biochemical and clinical features.[3] All patients were staged according to the Durie and Salmon classification system.[14] The parameters analyzed were: Age, gender, Durie and Salmon stage, presence and extent of lytic bone lesions, organomegaly, hemoglobin (Hb, g/L), total leukocyte count (×109/L), total platelet count (×109/L), serum creatinine (mg/dL), total protein (g/dL), albumin (g/dL), albumin to globulin ratio (A:G; < 1/>1), serum electrophoresis findings (cellulose acetate, pH = 8.6), corrected calcium (mg/dL), lactate dehydrogenase (LDH, IU/L) and light chain phenotype (ĸ or λ). Bone marrow trephine biopsy was fixed in 10% neutral buffered formalin, decalcified by sodium citrate-formic acid and then routinely stained with hematoxylin and eosin, Periodic acid Schiff and Grocott's silver impregnation technique. Wright-Giemsa stained bone marrow aspirate smears and trephine biopsy sections were then evaluated independently by three authors (SP, RGV, AR) for the myeloma cells (percentage of 500 nucleated cells); their cytomorphology (mature, small cell/lymphoplasmacytoid type - Grade I, intermediate/immature - Grade II, blastic/pleomorphic - Grade III); the presence or the absence of cytoplasmic (crystalline, Russell bodies) and/or intra-nuclear inclusions (Dutcher body); pattern of marrow infiltration (interstitial/diffuse/nodular/paratrabecular); histologic stage (extent of bone marrow infiltration by myeloma cells) (less than 20% - stage I, 20-50% - stage II, or >50% - stage III).[15] As per the protocol, 12 out of 14 patients received drugs such as thalidomide (Th), dexamethasone, bortezomib (Bz), melphalan, vincristine, doxorubicin/adriamycin or prednisolone in varying combinations; one received chemoradiotherapy; whereas one patient refused any definitive therapy. Th based regimen was used in 6, Bz in 3 and Th-Bz combination in two patients. The follow-up (n = 12) period ranged from 7 days to 34 months. The response to therapy was described as complete response (CR), partial response (PR), no response or progression of disease using the European group bone marrow transplantation criteria.[16]
Cyclin D1 IHC
Four micron thick deparaffinized bone marrow trephine biopsy sections were subjected to cyclin D1 IHC by manual method using rabbit monoclonal antibody to cyclin D1 (clone EPR2241, predilluted, Biogenex, Hyderabad, India) (avidin-biotin-peroxidase complex method). Antigen retrieval was done by prior heating the tissue sections in a Pascal pressure cooker in 0.01M citrate buffer (pH = 6) for 10-15 min. After the development of chromogen, all slides were counterstained with Hematoxylin. All three authors (SP, RGV, AR) who were blinded for the clinical features, evaluated the staining pattern under × 400 magnification in Olympus CX 41 microscope (Malaysia). As proposed by others,[13,17] only nuclear positivity, in at least 10% of myeloma cell nuclei, was considered as positive. The positive reaction was then graded semi-quantitatively as 1 + (10-19% nuclei positive), 2 + (20-50% nuclei positive) or 3 + (>50% nuclei positive); and each positive reaction was further characterized as strong, intermediate or weak intensity. Lymph node sections from a patient with mantle cell lymphoma were taken as a positive control for cyclin D1 IHC; whereas sinusoidal endothelial cell positivity to cyclin D1 (in bone marrow) served as positive internal control. The pattern of cyclin D1 expression was correlated with the clinico hematologic parameters.
Statistical analysis
Based on the pattern of expression, the patients were divided in two subgroups such as cyclin D1 positive (cyclin D1−) and cyclin D1 negative (cyclin D1−). Comparison of variables between two groups was performed by using independent t-test, Mann-Whitney U-test and Fisher's exact test. P ≤ 0.05 was considered to be statistically significant and Statistical Package for the Social Sciences-16 software (IBM SPSS Inc, Chicago, USA) was used for analysis.
RESULTS
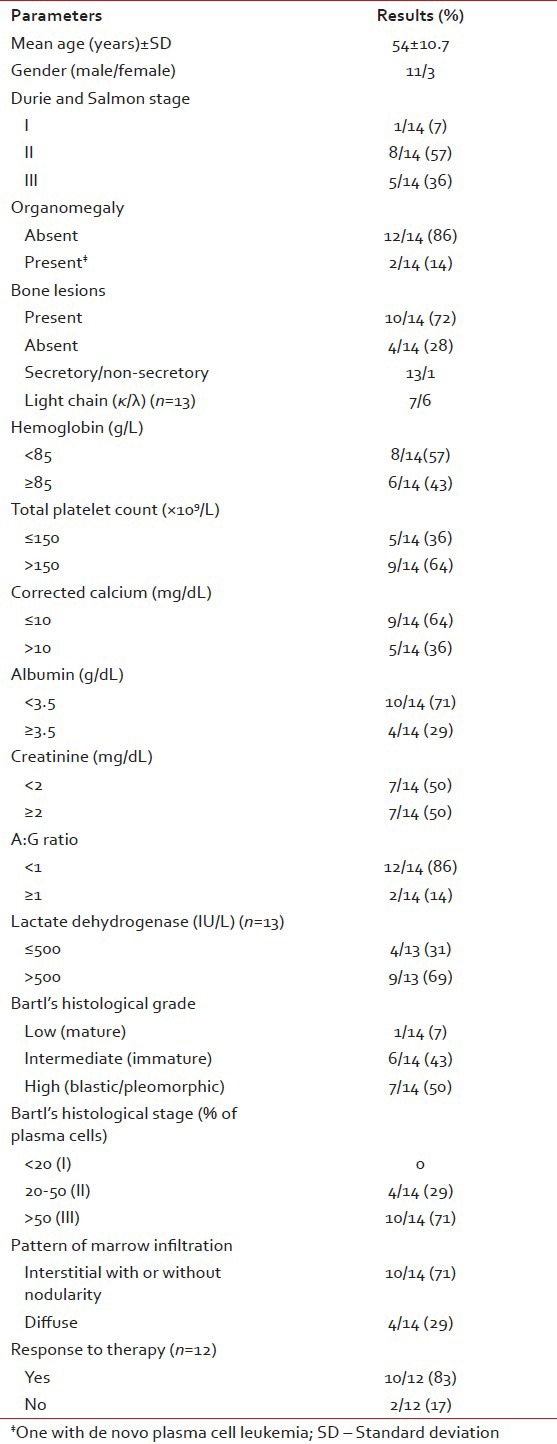
The clinicopathologic characteristics of all 14 patients with MM (13 newly diagnosed, 1 relapsed) are presented in Table 1. There were 11 males and 3 females with age range of 38-81 years (mean = 54 years, standard deviation = 10.7); and all were symptomatic at the time of evaluation. Eight (57%) were in Durie and Salmon Stage II, 5 (37%) Stage III and 1 (7%) in Stage I. Out of 14 cases, 10 (71.5%) presented with advanced lytic bone lesions, and two had organomegaly (one of which was de novo plasma cell leukemia). All, except one, had demonstrable light chain restriction (7 ‘ĸ’, 6 ‘λ’) by either IHC or biochemical assay techniques. Corrected serum calcium was found to be within normal range (≤11 mg/dL) in the majority of patients. On bone marrow evaluation, 10/14 (71%) cases showed an interstitial pattern of infiltration with or without focal nodularity; and 4 (29%) had packed marrow (diffuse pattern). Out of 14 cases, 10 (71%) cases were in Bartl's histologic Stage III (>50% tumor cells); and in 7 (50%), myeloma cells exhibited a high grade (blastic or pleomorphic) phenotype. Intracytoplasmic (Russell bodies) and intranuclear (Dutcher bodies) were noted in 11/13 patients; and in two intracytoplasmic crystalline inclusions were seen [Figures [Figures1a1a–g].
Table 1
Clinicopathological parameters of 14 patients of multiple myeloma (January 2011 to September 2012)
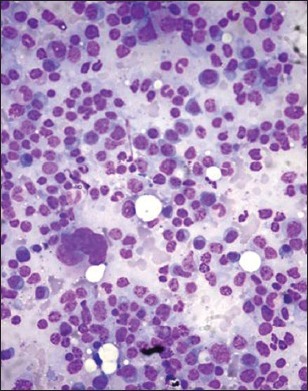
| Fig. 1a Bone marrow aspirate smears from patients with myeloma showing mature (grade 1) myeloma cells. Note the small cell/lymphoplasmacytic morphology in some (Wright-Giemsa, ×100)
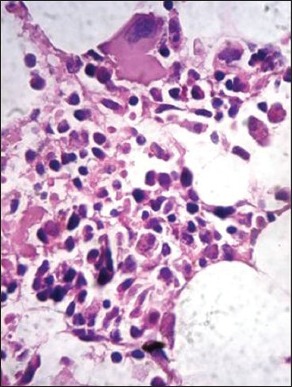
| Fig. 1g Presence of intracytoplasmic crystalline inclusions in myeloma cells (H and E, ×400)
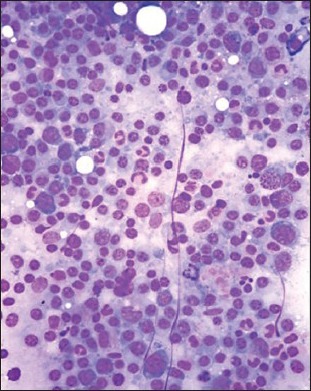
| Fig. 1b Bone marrow aspirate smears from patients with myeloma showing admixture of mature (grade 1) and immature myeloma cells (those with increased nuclear to cytoplasmic ratio, grade II) (Wright-Giemsa, ×400)
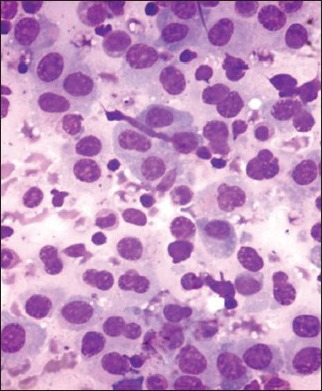
| Fig. 1c Bone marrow aspirate smears from patients with myeloma showing pleomorphic myeloma cells/plasmablasts (grade III). These cells are characterized by high nuclear to cytoplasmic ratio, polylobated nuclei with prominent nucleoli (Wright-Giemsa, ×400)
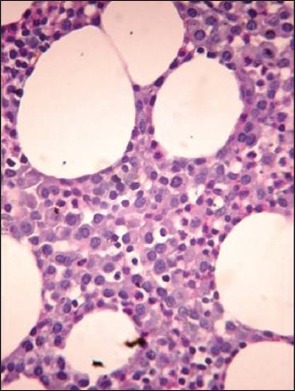
| Fig. 1d Hematoxylin and eosin stained bone marrow trephine sections showing interstitial pattern of infiltration by myeloma cells (×400)
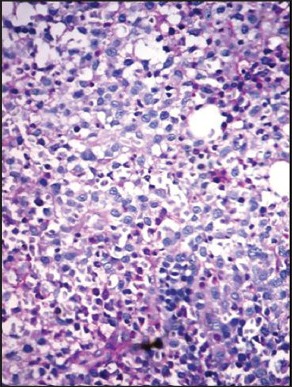
| Fig. 1e Periodic acid Schiff (PAS) stained bone marrow trephine sections showing a diffuse pattern of infiltration by myeloma cells (packed marrow) (×400)
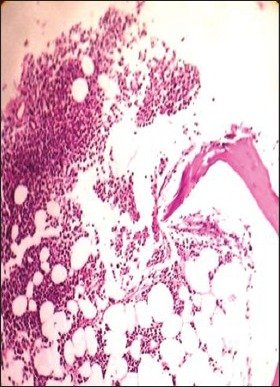
| Fig. 1f Hematoxylin and eosin stained bone marrow trephine sections showing a nodular pattern of infiltration by myeloma cells (×100)
Cyclin D1 nuclear expression was noted in 8/14 (57%) by means of IHC. In three cases (Case no. 1, 3, 7), the expression was of strong intensity, in ≥50% tumor cells; it was of intermediate to strong intensity in 20-50% cells in another three cases (Case no. 2, 6, 14); whereas weak nuclear positivity in 10-20% cells was observed in remainder (Case no. 4, 8) [Figures [Figures2a2a–d]. Of 8 cyclin D1+ patients, 4 (50%) showed CR (1 with strong intensity, 3 with intermediate to strong intensity of reaction), 2 (25%) had PR to chemotherapy (both with weak intensity of reaction); and 2 died of the disease (both with strong intensity reaction). On the other hand, 4/6 (67%) cyclin D1− patients showed PR, one refused any definite therapy and the other one was lost to follow-up [Table 2]. Among cyclin D1+ group, the duration of follow-up ranged from 7 days (Case 1, 3+ strong intensity reaction, expired) to 34 months (Case 2, 2+ strong intensity reaction, CR, alive) (mean = 9.4 months) compared to cyclin D1− group (follow-up duration; 10-18 months) (P > 0.05) [Tables [Tables2 and and3].
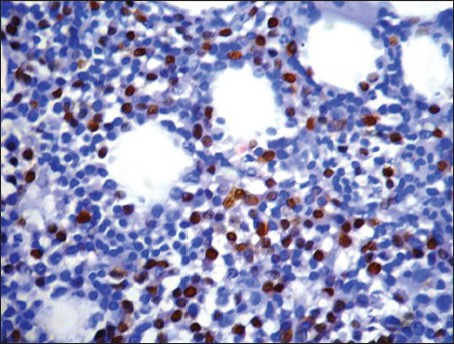
| Fig. 2a Pattern of cyclin D1 expression in multiple myeloma. Strong nuclear positivity in greater that 50% tumor cells (3+) (Peroxidaseantiperoxidase stain, ×400)
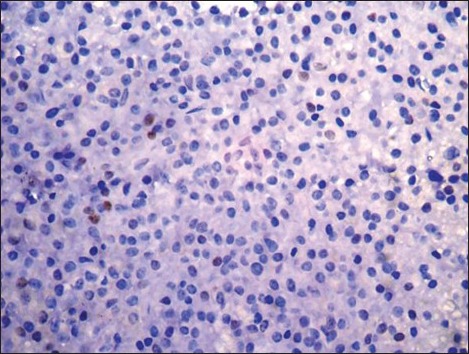
| Fig. 2d Weak nuclear positivity in less than 10% tumor cells (negative) (Peroxidase-antiperoxidase stain, ×400)
Table 2
Clinical and histologic stage, pattern of cyclin D1 expression and therapeutic outcome in 14 patients of multiple myeloma
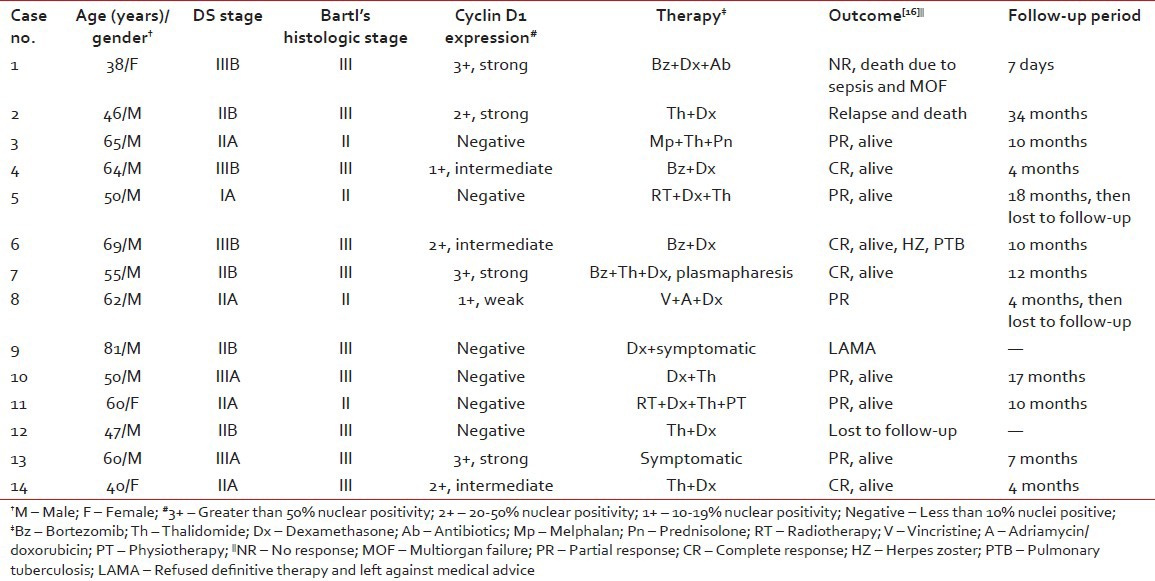
Table 3
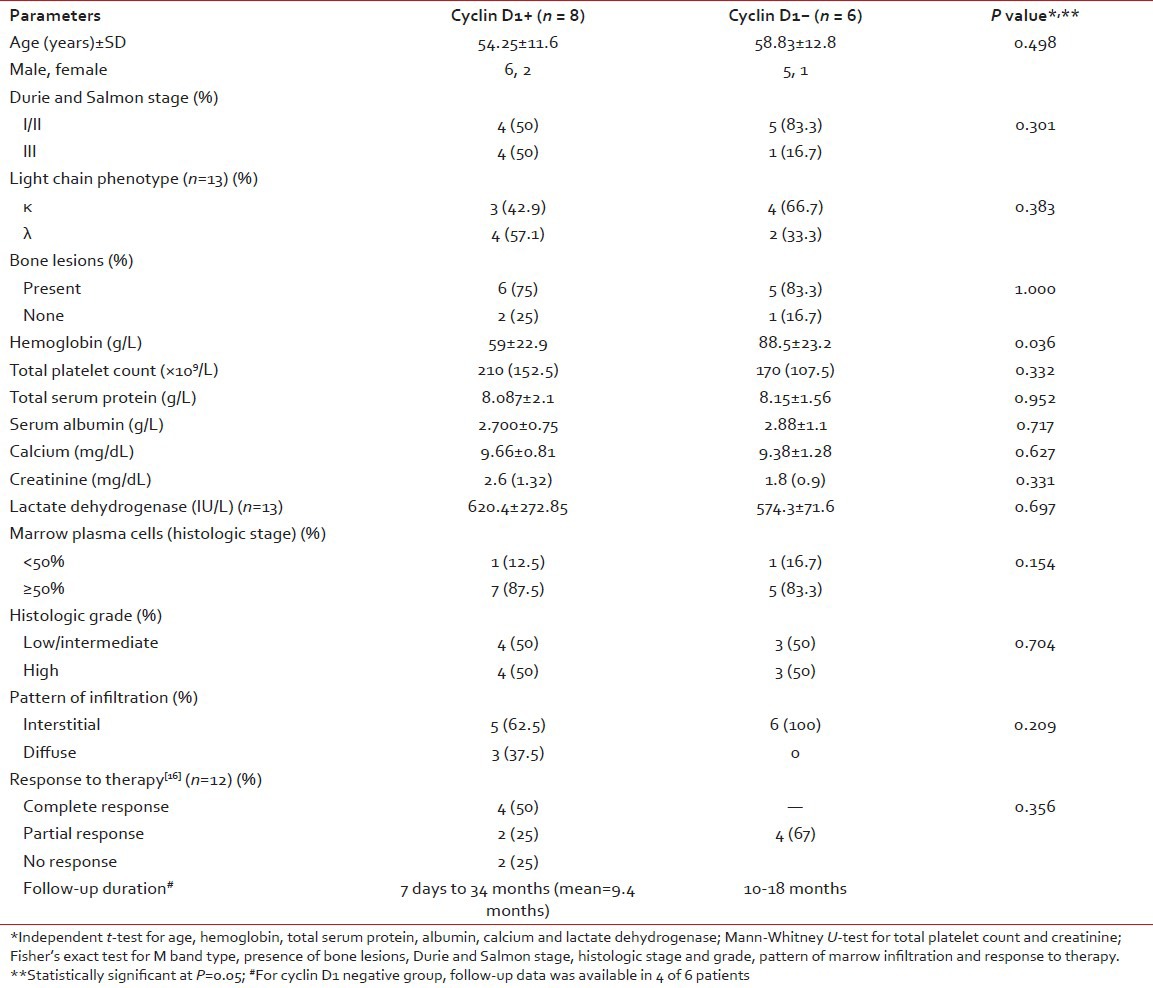
Relationship of cyclin D1 expression with clinicopathological parameters and response to therapy
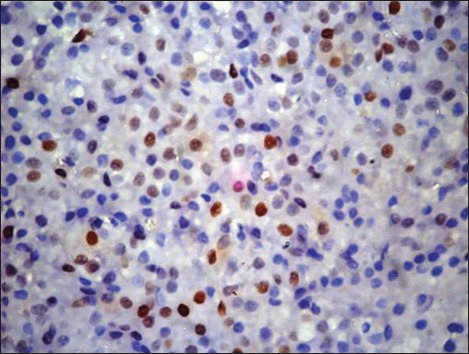
| Fig. 2b Intermediate to strong nuclear positivity in 30-50% tumor cells (2+) (Peroxidase-antiperoxidase stain, ×400)
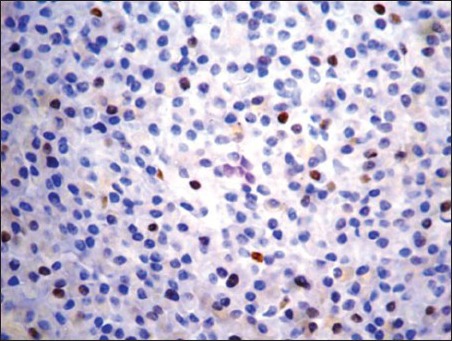
| Fig. 2c Weak to intermediate nuclear positivity in 10-20% tumor cells (1+) (Peroxidase-antiperoxidase stain, ×400)
Cyclin D1+ group had significantly lower Hb level (P = 0.03) than cyclin D1− group; though both groups showed no statistical significance (P > 0.05) in regard to age, gender, Durie and Salmon stage, lytic bone lesions, light chain phenotype, creatinine, calcium, LDH, leukocyte and platelet count and bone marrow histology [Table 3].
DISCUSSION
We observed a higher percentage of cyclin D1 [removed]57%) in patients with MM by using IHC on bone marrow trephine sections; and in 6/14 (43%), the results were negative. Recent studies have demonstrated that translocations involving the immunoglobulin heavy chain gene locus (14q32) are the most frequent chromosomal aberrations in MM. The partner chromosomes in most cases are 11q13 (in the region of the bcl-1 gene) and less commonly 8q24, 18q21.3, 4p16.3 and 16q23.[4,5] The negative expression in other six patients in our series, might be explained by the fact that pathogenetic events other than t (11;14) (q13;q32) such as RB gene deletion, abrogation of G1/S phase might be responsible. However, none of our cases were subjected to any molecular analysis to substantiate this finding. Besides these, we did not find any cyclin D1+ resident hematopoietic cells in any of the bone marrow tissue sections analyzed by IHC; thus suggesting the specificity of this analysis. Moreover, another advantage of cyclin D1 IHC is its ready availability in most of the laboratories at an affordable cost.
Recent studies have confirmed the impact of several molecular markers on survival of MM patients. Immunohistochemical staining for proteins implicated in MM pathogenesis is useful for the evaluation of the activity of the relative genes. Majority of MM patients have deregulated at least one of the cyclin genes (D1, D2, D3), suggesting that this may have prognostic value.[18] However, a correlation between cyclin D1 expression, clinical parameters, histologic stage and the degree of differentiation of neoplastic plasma cells (histologic grading) has been reported only in a small series of patients.[13,17,19,20,21,22,23,24]
With IHC, cyclin D1 expression may be detected up to 25-35% and in up to 45-50% cases by FISH/polymerase chain reaction/microarray techniques. The difference in level of expression, as observed by various authors, may be due to type of case selection (new or relapsed/refractory patients), cut-off percentage of nuclear positivity taken (≥10% in most series) and sensitivities of the assay technique used. In our series, strong to intermediate intensity of nuclear positivity was noted in six patients and in remainders two, it was weak in intensity. All were symptomatic at the time of evaluation; a higher proportion of them were in Durie-Salmon Stage II/III (8/5) with advanced lytic bone lesions (10/14); most of them had a preponderance toward immature (n = 6) to blastic phenotype (n = 7) and greater extent of marrow infiltration (10/14, Stage III); all indicative of increased tumor burden or progression of the disease process [Table 1]. Compared to cyclin D1− group (4/6 PR), 4/8 cyclin D1+ patients achieved CR, 2 had PR; and remainder 2 died of the disease; though there was discordance between the intensity of reaction and response to therapy [Table 2]. However, in view of a very small series of patients and smaller follow-up period, the outcome of analysis was statistically insignificant [Table 3].
A comparative review of literature describing the correlation between cyclin D1 expression and clinicopathological features in myeloma is presented in Table 4. Previous studies have described a strong positive association between cyclin D1 expression with advanced clinical stage, higher histologic stage and grade, increased proliferative activity (plasma cell labeling index), impaired renal functions and higher β2 microglobulin levels.[10,11,17,20,21,22,23,24] However, other studies in this context have shown conflicting results.[8,13,19] A recent study,[8] recruited including 94 myeloma patients, and found no difference in the pattern of expression in a newly diagnosed (n = 49) and relapsed/refractory (n = 45) myeloma which was consistent with the observation that nearly all subsets of myeloma eventually relapse with current treatment protocol. Moreover, no significant association was found with the degree of expression and clinicopathologic features in that study. Similarly, in another series of 59 newly diagnosed myeloma patients, cyclin D1 positivity or negativity did not convey any significant correlation with clinicopathological features or survival.[19] Hoyer et al. analyzed 24 cases of MM, of which 10 had a lymphoplasmacytoid morphology and 4 were in leukemic phase. On IHC, strong cyclin D1 positivity was noted in 19/24 and all 19 patients died with the disease.[21]
Table 4
Review of literature describing the correlation between cyclin D1 expression and clinicopathologic features in patients of multiple myeloma
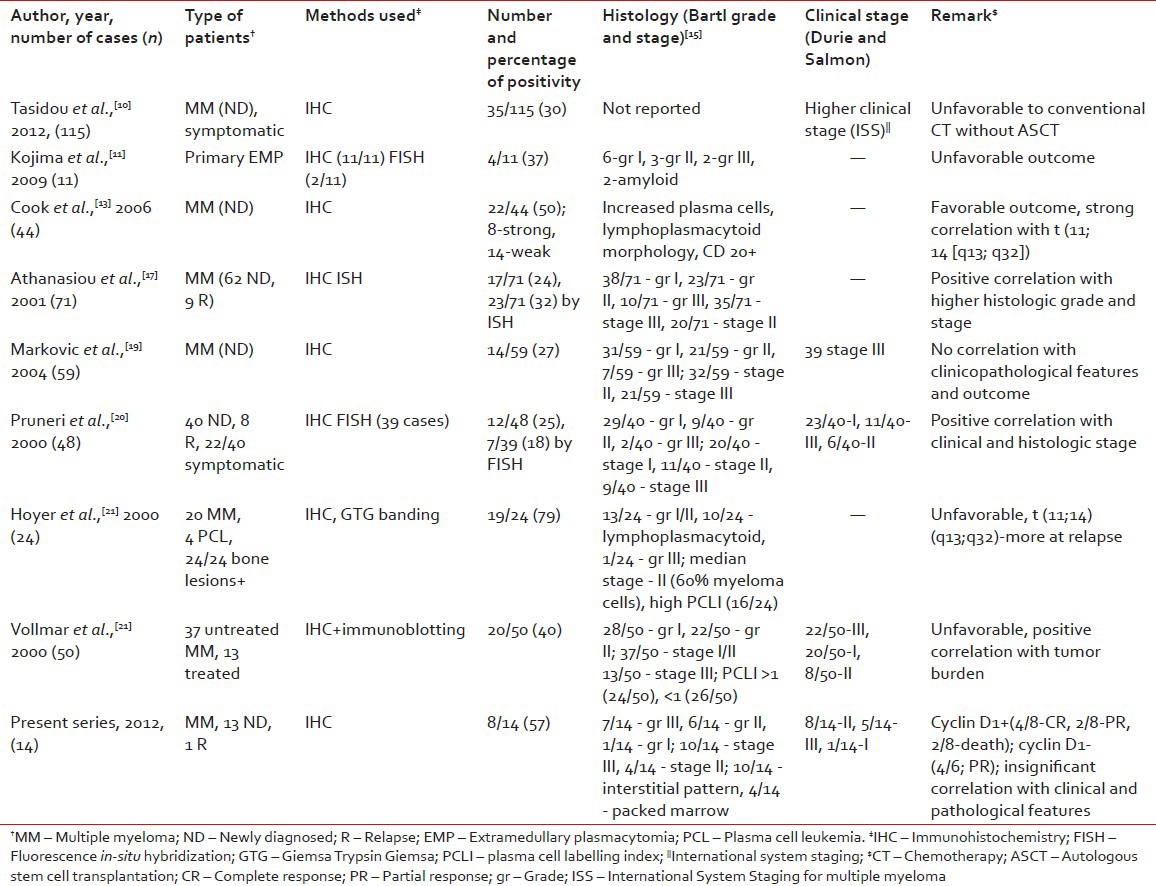
Cook et al. described two prognostically favorable subgroups of cyclin D1+ myeloma patients by means of IHC and FISH.[13] Strong cyclin D1 immunostaining was associated with a higher percentage of myeloma cells at diagnosis (median = 60%), distinct lymphoplasmacytoid morphology, strong CD20 expression and t (11; 14) (q13; 32) translocation (by FISH). In contrast, weak staining was associated with hyperdiploid karyotype and gain of CCND1 locus. Although both subgroups showed distinct histomorphological patterns, both experienced a superior/favorable survival over cyclin D1-group (3 year expected survival 73% vs. 27%, respectively, P = 0.005).
The pathobiology of extramedullary plasmacytomas (EMP) may be quite distinct from MM. A recent Japanese study evaluated immunohistochemical expression of cyclin D1 in a series of 11 primary EMP (none with myelomatous transformation at the time of analysis).[11] Out of 11 cases, 4 (37%) were cyclin D1+ (>30% tumor cell nuclei) and it was negative in remaining 7 cases. Five of 7 cyclin D1− patients showed a well-differentiated morphology and all 7 achieved CR. In contrast, 2/4 cyclin D1+ cases showed high grade morphology and 3 died of the disease. This finding suggests that cyclin D1 expression may be an adverse prognostic indicator in EMP. However, most of the patients, in that series, received radiotherapy, either solely (5/11) or in combination with surgery/chemotherapy, which might have influenced the outcome.
The introduction of autologous stem cell transplantation (ASCT) and novel agents such as immunomodulators (Th, lenalidomide) and proteasome inhibitors like Bz have changed the management of myeloma patients and extended the overall survival (OS).[25] Cyclin D1, D2 and D3 proteins are regulated by ubiquitin-proteasome mediated degradation. Cyclin D1 is rapidly degraded by the 26S proteasome and Bz increases its activity in vivo. Cyclin D1 levels inversely correlate with STAT-3 levels, resulting in downstream effects that increase the susceptibility of cells to apoptosis.[12] Overexpression of cyclin D1 correlated with a durable response to and better prognosis to Bz therapy in small series of patients.[12,26] On the other hand, cyclin D1 expression conveyed an unfavorable prognosis in 115 newly diagnosed patients of symptomatic myeloma treated with Bz and/or Th based regimens; although ASCT were offered to a lesser number of patients.[10] Another study evaluated the prognostic significance of cyclin kinase (CK) 1B nuclear expression in 60 relapsed/refractory myeloma patients by IHC and FISH.[9] There was no significant difference in response rate between patients with (19/60) and without (41/60) CK 1B expression. However, patients with CK 1B expression had significant shorter progression free survival (PFS) and OS compared to those without CK 1B. In contrast, other studies did not find the prognostic significance of cyclin D1 and other markers on PFS and OS in newly diagnosed or relapsed/refractory myeloma treated with Th based regimen.[8] The discrepancy between above results may be explained by the difference in the study population (newly diagnosed or relapsed/refractory), therapeutic regimen used (with or without ASCT) and the duration of follow-up.
SUMMARY AND FUTURE PERSPECTIVE
Our observation suggests that cyclin D1 IHC performed on an adequate, appropriately processed trephine biopsy specimen may be a sensitive tool for the prognostic evaluation of myeloma patients in a resource poor setting and only negative results should be confirmed by molecular studies. Furthermore, cyclin D1 expression may have a favorable or at least, neutral prognostic impact on a subset of patients with myeloma. Small sample size, lack of correlation with molecular studies due to financial constraints and shorter duration of follow-up, were the major drawbacks of our study. Therefore, larger prospective studies in the near future is required to validate our observations; to compare the pattern of expression between newly diagnosed and at follow-up (after 6 months), as well as among newly diagnosed and relapsed patients.
ACKNOWLEDGMENT
The authors sincerely thank Dr. Prathiba, M.D., Professor and Head of the Department of Pathology, Sri Ramachandra Medical University, Porur, Chennai, India, for her technical help in performing cyclin D1 IHC on bone marrow trephine sections; and Miss Bridgitte Ambrose of Pondicherry Institute of Medical Sciences for the statistical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1a Bone marrow aspirate smears from patients with myeloma showing mature (grade 1) myeloma cells. Note the small cell/lymphoplasmacytic morphology in some (Wright-Giemsa, ×100)

| Fig. 1g Presence of intracytoplasmic crystalline inclusions in myeloma cells (H and E, ×400)

| Fig. 1b Presence of intracytoplasmic crystalline inclusions in myeloma cells (H and E, ×400)

| Fig. 1c Bone marrow aspirate smears from patients with myeloma showing pleomorphic myeloma cells/plasmablasts (grade III). These cells are characterized by high nuclear to cytoplasmic ratio, polylobated nuclei with prominent nucleoli (Wright-Giemsa, ×400)

| Fig. 1d Hematoxylin and eosin stained bone marrow trephine sections showing interstitial pattern of infiltration by myeloma cells (×400)

| Fig. 1e Periodic acid Schiff (PAS) stained bone marrow trephine sections showing a diffuse pattern of infiltration by myeloma cells (packed marrow) (×400)

| Fig. 1f Hematoxylin and eosin stained bone marrow trephine sections showing a nodular pattern of infiltration by myeloma cells (×100)

| Fig. 2a Pattern of cyclin D1 expression in multiple myeloma. Strong nuclear positivity in greater that 50% tumor cells (3+) (Peroxidaseantiperoxidase stain, ×400)

| Fig. 2d Weak nuclear positivity in less than 10% tumor cells (negative) (Peroxidase-antiperoxidase stain, ×400)

| Fig. 2b Intermediate to strong nuclear positivity in 30-50% tumor cells (2+) (Peroxidase-antiperoxidase stain, ×400)

| Fig. 2c Weak to intermediate nuclear positivity in 10-20% tumor cells (1+) (Peroxidase-antiperoxidase stain, ×400)


 PDF
PDF  Views
Views  Share
Share

