Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 107-111
DOI: DOI: 10.4103/0971-5851.99746
Abstract
Context: The diagnosis of papillary thyroid carcinoma (PTC) is based on nuclear features. These features may be present in focal areas in benign thyroid diseases and follicular adenoma (FA), leading to diagnostic difficulty. Aims: To evaluate the expression and pattern of the distribution of cytokeratin 19 (CK19) in PTC and compare its reactivity with other neoplastic and non-neoplastic conditions to assess its potential as a useful marker for PTC. Materials and Methods: Twenty two cases of papillary carcinoma (usual type, follicular and diffuse sclerosing variant), eight follicular adenomas, eight multinodular goiters (MNG) were collected for a period of two years and six months. Sections were taken from thyroidectomy specimens fixed in 10% buffered neutral formalin. Hematoxylin and eosin staining and immunohistochemical staining for CK19 were done using standard protocol. Results were semiquantitatively scored as follows: 1+ ( < 5% positively stained cells), 2+ (5-25%), 3+ (25-75%) and 4+ (>75%), and then analyzed. Statistical Analysis and Results: All 22 (100%) papillary carcinomas showed diffuse and strong (3+ and 4+) CK19 expression. Six out of eight (75%) FAs and four out of eight (50%) MNG were positive for CK19, but it was of weaker intensity (1+ and 2+) and focal in distribution. Conclusion: Focal CK19 staining may be found in benign disease, but diffuse and strong positivity is characteristic of PTC, which can be used in the diagnosis of PTC in lesions of equivocal morphological appearances.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
The diagnosis of papillary thyroid carcinoma (PTC) is based on nuclear features. These features may be present in focal areas in benign thyroid diseases and follicular adenoma (FA), leading to diagnostic difficulty.
Aims:
To evaluate the expression and pattern of the distribution of cytokeratin 19 (CK19) in PTC and compare its reactivity with other neoplastic and non-neoplastic conditions to assess its potential as a useful marker for PTC.
Materials and Methods:
Twenty two cases of papillary carcinoma (usual type, follicular and diffuse sclerosing variant), eight follicular adenomas, eight multinodular goiters (MNG) were collected for a period of two years and six months. Sections were taken from thyroidectomy specimens fixed in 10% buffered neutral formalin. Hematoxylin and eosin staining and immunohistochemical staining for CK19 were done using standard protocol. Results were semiquantitatively scored as follows: 1+ ( < 5% positively stained cells), 2+ (5-25%), 3+ (25-75%) and 4+ (>75%), and then analyzed.
Statistical Analysis and Results:
All 22 (100%) papillary carcinomas showed diffuse and strong (3+ and 4+) CK19 expression. Six out of eight (75%) FAs and four out of eight (50%) MNG were positive for CK19, but it was of weaker intensity (1+ and 2+) and focal in distribution.
Conclusion:
Focal CK19 staining may be found in benign disease, but diffuse and strong positivity is characteristic of PTC, which can be used in the diagnosis of PTC in lesions of equivocal morphological appearances.
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common form of malignant thyroid neoplasm. Its diagnosis is based on nuclear features such as nuclear clearing, overlapping, grooves and pseudoinclusions.[1] However, identification of these features remains, at times, difficult because of its focal presence and thus the distinction of PTC from other thyroid lesions may not be possible. Several variants of PTC have been described. Of these, Follicular variant of papillary thyroid carcinoma (FVPTC) is the most common one, which is characterized by an almost exclusive follicular growth pattern and a set of nuclear features identical to those of the Usual type of papillary thyroid carcinoma (UTPTC). Diagnostic dilemma may arise when an encapsulated nodule with a follicular pattern of growth exhibits clear nuclei with grooves or darkly staining colloid and distinguishing follicular adenoma (FA) from encapsulated FVPTC becomes difficult.[2]
There are several other thyroid lesions that may contain papillary processes with nuclear features in a focal manner, which pose diagnostic difficulties with PTC. Multinodular goiter (MNG) with delicate papillary budding and focal nuclear clearing may be confused with PTC.[3]
Several immunohistochemical stains have been investigated for their possible role as diagnostic markers for PTC. They are cytokeratin19 (CK19), HBME1, galectin-3 and RET and thyroid transcription factor 1.[4–9] Although galectin-3 was initially shown to have utility in the differential diagnosis between benign and malignant thyroid lesions,[10,11] recent studies suggest that it is not reliable.[12–14] Several studies have shown conflicting results regarding the usefulness of CK19 as a diagnostic marker in PTC.[13–20] This study was carried out to investigate the role of CK19 as a possible diagnostic marker of PTC and its utility in differentiating PTC from the above-mentioned thyroid lesions.
MATERIALS AND METHODS
Forty-two cases were collected for a period of 2 years and 6 months. Hematoxylin-eosin stain was performed for tumor diagnosis and histologic types. Immunohistochemical stains were performed on 5-μm-thick paraffin sections taken on poly-L-lysine-coated slides. Enzyme pretreatment was done for CK19 staining by incubating the sections with pepsin at 37°C for 5 min for antigen retrieval. Controls were performed and stained appropriately. Extent of CK19 staining was semiquantitatively scored as follows [Table 1].[20]
Table 1
Semi quantitative scoring of CK19 expression according to the percentage of positively stained cells

All the statistical analyses were performed with stastistica version 6.0 (Statsoft Inc.; 2001, Tulsa, OK, USA) and Windows software. Approval from local Institutional Ethics Committee was obtained.
RESULTS
A total of 38 cases of thyroidectomy specimens were selected for the study. Twenty-two cases were PTC, of which eight cases were follicular variant PTC, a single case was diffuse sclerosing variant and the remaining 13 were UTPTC. There were eight cases each of MNG and FA.
Regarding age distribution, on applying the non-parametric Kruskal-Wallis test in the three groups (PTC, MNG and FA), there was no difference in median age distribution. Median age was taken because there was variation in the number of samples. On analyzing the sex distribution of PTC, it was observed that 19 cases (86.36%) were females and three cases (13.64%) were males, with a male:female ratio of 1:6.3. In MNG and FA, the male:female ratios were 1:7 and 1:3, respectively.
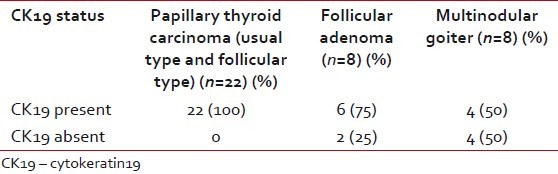
CK19 expression was seen in all 22 PTC (100%) in a diffuse manner, whereas only four cases of MNG and six cases of FA showed focal expression. This observation was highly significant (Chi-square test 2-tailed P value=0.003) [Table 2].
Table 2
Presence/absence of cytokeratin 19 expression in papillary thyroid carcinoma, follicular adenoma and multinodular goiter
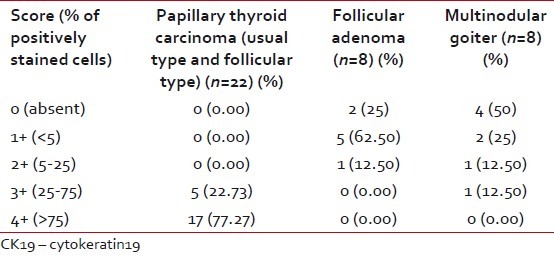
When CK 19 semiquantitative score was analyzed, 17 out of 22 (77.27%) PTC showed 4+ positivity and the remaining five (22.73%) expressed 3+ positivity. All showed diffuse positivity. Only four cases out of eight MNG (50%) focally expressed CK19, two with 2+, one with1+ positivity and only one showed 3+ positivity. Six cases out of eight FA (75%) were also focally positive for CK19, five (62.50%) with 1+ positivity and a single case showed 2+ positivity. Taking CK19, 4+ as a cut-off value for positivity for detecting PTC versus MNG and FA, there was 77% sensitivity with a very high specificity and positive predictive value each of 100% [Table 3].
Table 3
Semiquantitative expression of CK19 in papillary thyroid carcinoma, follicular adenoma and multinodular goiter
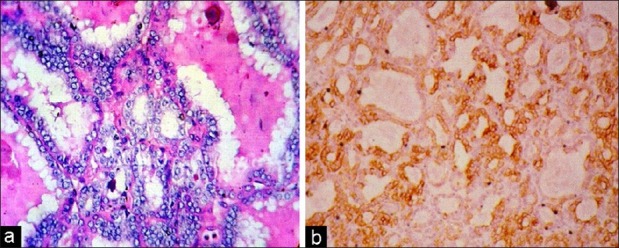
Comparing usual type PTC and follicular variant PTC, it was observed that in UTPTC, 4+ positivity was seen in 11 of 13 cases (84.62%) and the remaining showed 3+ positivity [Figure 1]. In cases of FVPTC, it was found that five of eight (62.50%) showed 4+ positivity and the remaining three showed 3+ positivity (37.50%) [Figure 2]. Applying the Fisher exact test, there was no significant difference between the semiquantitative scores of UTPTC and FVPTC. Seventy-five percent of FA showed CK19 focal expression of weak intensity [Figure 3]. As there was no. 3+ or 4+ expression in FA and all the cases of FVPTC showed either 3+ or 4+ CK19 expression, using the non-parametric Chi-square test, the 2-sided P value was found to be 0.001, which was highly significant. All the cases of UTPTC showed either 4+ or 3+ expression, whereas FA showed only 1+ or 2+ positivity. Thus, semiquantitative CK19 score was found to be useful to differentiate FA from both UTPTC and FVPTC [Table 4].
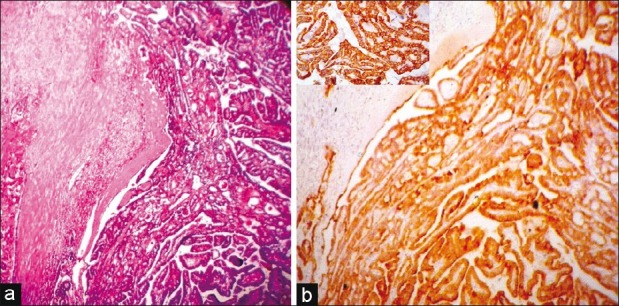
| Fig. 1 (a) A usual type papillary thyroid carcinoma (hematoxylineosin, ×LP), (b) A usual type papillary thyroid carcinoma, immunostaining for cytokeratin 19-diffuse and strong (4+) positive (IHC, ×LP, inset HP)
| Fig. 2 (a) A follicular variant papillary thyroid carcinoma with typical nuclear features (hematoxylin-eosin, ×HP) (b) A follicular variant papillary thyroid carcinoma, immunostaining for cytokeratin 19 (IHC, ×HP)
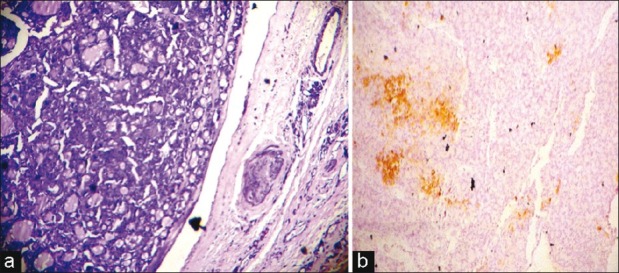
| Fig. 3 (a) Photomicrograph of a follicular adenoma (hematoxylineosin, ×LP) (b) A follicular adenoma, immunostaining for cytokeratin 19, focal positive (1+) (IHC, ×LP)
Table 4
Semiquantitative expression of CK19 in usual type papillary thyroid carcinoma, follicular variant papillary thyroid carcinoma, follicular adenoma and multinodular goiter
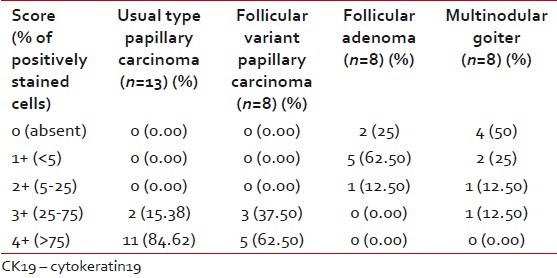
In MNG, only 50% (4/8) cases showed CK19 expression. Only one case of MNG showed 3+ positivity and all the remaining showed either 1+ or 2+ positivity (three cases) or negative staining (four cases) [Figure 4]. However, 84.62% (11/13) of UTPTC expressed 4+ positivity and the remaining 15.38% showed 3+ positivity. Applying Chi-square test, the 2-sided P value was found to be < 0.001, which was highly significant. Similar to FA, the semiquantitative score of CK19 of MNG was useful to differentiate it from both UTPTC and FVPTC [Table 4].
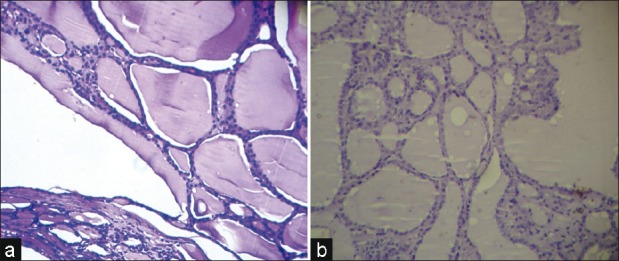
| Fig. 4 (a) Photomicrograph of multinodular goiter (hematoxylineosin, ×LP) (b) A multinodular goiter, negative immunostaining for cytokeratin 19 (IHC, ×LP)
DISCUSSION
With an age range of 15–53 years, the median age of PTC was 34.50 years in this study, which was similar to the study done by Mazzaferri et al.[21] and Preston-Martin et al.[22] They found that most of the PTC were diagnosed in the third to fifth decades. Sahoo et al.,[20] showed a higher mean age of 46 years, with an age range of 33-78 years in their five cases of UTPTC, which may be explained by the small number of cases compared with our study.
In this study, the male: female ratio, in case of PTC, was 1:6.3, which was slightly more than that already published in the literature. According to Negri et al.,[23] PTC was about four-times more common in females than in males, and this was also seen in the study done by Mazzaferri et al.[21] This excessive female predominance may be due to the small number of cases in our study. However, Sahoo et al.,[20] have shown a male:female ratio of 1:9 in their 10 cases of FVPTC, which is slightly higher than our findings of 1:6.
The role of cytokeratin 19 in the diagnosis of PTC is not yet fully established. Different studies have shown varying percentage distribution of CK19 positivity in PTC and other lesions of the thyroid. In this study, all 22 cases of PTC expressed CK19 in a diffuse and strong manner. Fifty percent (4/8) of MNG and 75% (6/8) of FA also showed immunoreactivity to CK19, except one case of MNG. In all of these cases, expression was focal and weak in intensity. Only one case of MNG out of eight was strongly positive, but only in focal areas. The significance of this expression was unexplainable. The study by Nasr et al.[15] also noted a high rate of CK19 positivity in benign lesions in as much as 68% (39/57), but staining intensity was weak in these benign lesions. In their study, three out of four papillary hyperplastic nodules were negative for CK19, whereas focal staining was present in one. Fifty percent (5/10) of their cases of MNG were also CK19 positive. They suggested that combination of HBME1 and CK19 attained a high sensitivity and specificity for the diagnosis of PTC.
Other studies showed a high sensitivity and specificity of CK19 in PTC. Baloch et al.[13] showed that CKl9 stained strongly and diffusely in all the cases of 10 UTPTC and all 26 FVPTC. Fonseca et al.,[24] in a similar study on paraffin-embedded material, found that CK19 was strongly expressed in papillary carcinoma and focally in follicular carcinoma. Miettinen et al.,[5] in their study of keratin subsets on paraffin-embedded papillary and follicular thyroid lesions, confirmed that CK19 is a useful marker for differentiating between papillary carcinoma and papillary hyperplasia. However, they also identified expression of CK19 in follicular neoplasms and, hence, this particular cytokeratin was alone not useful in differentiating it from follicular tumors.
If CK19 4+ positivity was taken as a cut-off value to differentiate PTC from other mimicking lesions, it showed 77% sensitivity and 100% specificity and positive predictive value in this study. Similar findings were observed in other studies also. Sahoo et al.,[20] noted similar findings of CK19 positivity in all PTC. They showed that 93% of PTC had 4+ positivity. In a large study, Cheung et al.,[7] reported diffuse and moderate to strong CK19 staining in 80% (43/54) of PTCs and 57% (48/84) of FVPTC. They also observed that 17% (6/35) of FA showed focal staining with CK19, and a single case (1/35) showed diffuse CKI9 staining. In addition, they showed that immunoreactivity for HBME-1 and RET represents a reliable indicator of malignancy in nodules of follicular epithelial derivation. However, unlike other studies, they did not estimate the percentage of positively stained cells. According to Kragsterman et al.,[14] all 35 patients (100%) with thyroid papillary carcinoma were positive for CK19 and in 80%, more than half of the tumor cells were immunostained, and the intensity of the immunoreactivity was strong. Guyetant et al.[18] noted similar findings with 16 usual-type PTCs and 43 FVPTC. CK19 was diffusely expressed in all their UTPTC and FVPTC. According to them, sensitivity and specificity of CK19 as a marker of papillary carcinomas was 100% and 82.5%, respectively. Beesley et al.[16] got similar results with diffuse and strong expression of CK19 in all four FVPTC (100%) and 20 out of 22 UTPTC (91%).
In this study, most of the UTPTC (84.62%) were diffuse 4+ positive. In case of FVPTC, 4+ was observed in 62.50%. According to Sahoo et al.,[20] 93% of FVPTC and all cases of UTPTC showed strong 4+ positivity for CK19. The study done by Cheung et al.[7] observed that 48 cases of FVPTC out of 84 (57%) were positive for CK19, and that this was diffuse and moderate to strong in intensity, resembling our 3+ and 4+ positivity. Baloch et al.[13] observed 26 cases of FVPTC with multifocal distribution of papillary cancer nuclei and 10 cases of usual type PTC; CKl9 stained strongly and diffusely in all cases of papillary carcinoma, and FVPTC cases showed strong staining of the areas with papillary cancer nuclei in all cases and moderate to strong staining in areas of tumor without obvious nuclear features of papillary cancer. Similar to this study, Beesley et al.[16] and Nasr et al.[15] found no difference in CK19 expression between UTPTC and FVPTC.
In this study, 50% of MNG were positive for CK19 expression, but only one case showed 3+ positivity. Miettinen et al.[5] observed focally strong positivity in occasional cases of MNG. Beesley et al.[16] found that CK19 expression was seen in two out of eight MNG with only focal staining. Nasr et al.[15] noted that only one out of four papillary hyperplastic nodules was positive, and only 15% of the cells stained, with the staining intensity being weak in most of these benign lesions. Cheung et al.[7] demonstrated that 20% (8/40) of the nodular goiters were focally CK19 positive, and immunoreactivity was seen mainly in areas of degeneration, indicating the reactive nature of CK19 positivity. It was evident from this study that 4+ immunoreactivity was helpful in distinguishing PTC from MNG. When studied separately, both FVPTC and UTPTC maintained a similar status of significance.
In our study, 75% of FA showed CK19 positivity. Sahoo et al.[20] also found CK19 positivity in 100% (20/20) of FA; of these, 75% showed 1+ positivity. However, in 25% of the FA, CK19 immunoreactivity was more extensive (2+ in one and 3+ in four), although none was 4+. In all these cases, CKI9 staining was patchy and often restricted to follicular cells located at the periphery of the nodule or those lining the cystic follicles. The intensity of staining was moderate. We did not find any 3+ or 4+ positive FA. Nasr et al.[15] also demonstrated the CK19 status in five out of six FAs, and the staining intensity was weak. Beesley et al.[16] observed that only five out of 20 FAs were positive for CK19, and this was in a focal distribution. Guyetant et al.[18] showed that 90% of the FAs (26/29) and four out of 11 atypical adenomas were focally positive for CK19. Low-CK19 expression was also observed by Rorive et al.[17] in the FAs as compared with PTC. The significance of focal expression of CK19 in some FA is unknown. Further studies are necessary to show whether these tumors have a different clinical behavior or molecular profile.
In our study, we found that diffuse and strong expression of CK19 is characteristic of PTC, both usual type and follicular variant. Although weak CK19 staining is common in MNG and FAs, a negative stain is a good evidence against PTC.
ACKNOWLEDGMENT
The authors are particularly thankful to their technical staff members, Janababu, Ashimda and Ratnadi, who actively helped in their work. The authors also owe them special appreciation for their cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 (a) A usual type papillary thyroid carcinoma (hematoxylineosin, ×LP), (b) A usual type papillary thyroid carcinoma, immunostaining for cytokeratin 19-diffuse and strong (4+) positive (IHC, ×LP, inset HP)

| Fig. 2 (a) A follicular variant papillary thyroid carcinoma with typical nuclear features (hematoxylin-eosin, ×HP) (b) A follicular variant papillary thyroid carcinoma, immunostaining for cytokeratin 19 (IHC, ×HP)

| Fig. 3 (a) Photomicrograph of a follicular adenoma (hematoxylineosin, ×LP) (b) A follicular adenoma, immunostaining for cytokeratin 19, focal positive (1+) (IHC, ×LP)

| Fig. 4 (a) Photomicrograph of multinodular goiter (hematoxylineosin, ×LP) (b) A multinodular goiter, negative immunostaining for cytokeratin 19 (IHC, ×LP)


 PDF
PDF  Views
Views  Share
Share

