Deferasirox in Indian children with thalassemia major: 3 years experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(01): 16-20
DOI: DOI: 10.4103/0971-5851.113407
Abstract
Objective: To evaluate the efficacy and safety of the oral iron chelator deferasirox in treating transfusional hemosiderosis in a cohort of Indian children with thalassemia major with high iron load. Materials and Methods: The first 50 children (age 2-18 yrs) with thalassemia major to commence deferasirox at our center were enrolled and followed up for a period of 36 months between April 2008 and March 2011. The dose of deferasirox was determined by their baseline serum ferritin and was adjusted to a maximum of 40 mg/kg/day depending on response. Ferritin levels, SGOT, SGPT, serum creatinine and urine albumin were regularly monitored. Results: Of the 50 patients, 76% documented a significant decline in serum ferritin ( P < 0.05). Seven (14%) patients had a stable ferritin whilst 5 patients (10%) documented an increase over the study period. The mean serum ferritin at baseline, 12, 24 and 36 months was 4354, 3260, 3290 and 3042, respectively ( P < 0.05). The median serum ferritin at the same time points was 3555, 2810, 2079 and 2271, respectively ( P < 0.05). No severe toxicity was seen. Conclusions: Deferasirox, when given in doses ≥30 mg/kg, was found to be an effective and safe drug in reducing transfusional hemosiderosis. Thirty five (70%) needed dose escalation upto 40 mg/kg/day. Fifteen (30%), however did not achieve a negative iron balance despite maximally permissible doses.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objective:
To evaluate the efficacy and safety of the oral iron chelator deferasirox in treating transfusional hemosiderosis in a cohort of Indian children with thalassemia major with high iron load.
Materials and Methods:
The first 50 children (age 2-18 yrs) with thalassemia major to commence deferasirox at our center were enrolled and followed up for a period of 36 months between April 2008 and March 2011. The dose of deferasirox was determined by their baseline serum ferritin and was adjusted to a maximum of 40 mg/kg/day depending on response. Ferritin levels, SGOT, SGPT, serum creatinine and urine albumin were regularly monitored.
Results:
Of the 50 patients, 76% documented a significant decline in serum ferritin (P < 0.05). Seven (14%) patients had a stable ferritin whilst 5 patients (10%) documented an increase over the study period. The mean serum ferritin at baseline, 12, 24 and 36 months was 4354, 3260, 3290 and 3042, respectively (P < 0.05). The median serum ferritin at the same time points was 3555, 2810, 2079 and 2271, respectively (P < 0.05). No severe toxicity was seen.
Conclusions:
Deferasirox, when given in doses ≥30 mg/kg, was found to be an effective and safe drug in reducing transfusional hemosiderosis. Thirty five (70%) needed dose escalation upto 40 mg/kg/day. Fifteen (30%), however did not achieve a negative iron balance despite maximally permissible doses.
INTRODUCTION
The advent of effective chelation therapy in 1960s was a major milestone in the treatment of transfusion-dependent patients. Deferoxamine (DFO), a hexadentate chelator was introduced in 1970's and has been the standard of care until recently and had led to dramatic strides in the survival of thalassemia patients.[1] However the parenteral mode of administration and cost has hindered optimal compliance. Deferiprone, a bidentate chelator has been in use since 1987 and its efficacy, especially, in removing myocardial iron has been documented extensively.[2] However, side effects such as erosive arthritis in 5-20%, neutropenia in up to 5% of patients and severe agranulocytosis in up to 0.5% of patients mandate very close monitoring.[2,3]
The ideal iron-chelating agent should have a number of properties in addition to its ability to bind iron, which include good oral bioavailability and once-daily dosing regimen. Deferasirox (ICL670) belongs to a new class of oral tridentate chelator, N-substituted bis-hydroxyphenyltriazoles and is the result of a concerted discovery program. Its efficacy and safety has been demonstrated in many preclinical,[4] phase I and phase II studies in adult[5,6] and pediatric[7] thalassemics. With a plasma half-life of 8 to 16 hours, once-daily dosing permits circulating drug at all times to scavenge non-transferrin-bound labile plasma iron.[8]
Capellini et al.[9] in a trial involving nearly 600 patients showed the equivalent efficacy of deferasirox to deferoxamine. Importantly, it was clearly seen that the response was dose dependent and to achieve a reduction in serum ferritin, a dose of at least 30 mg/kg/day was required. There is also clear evidence from both phase 2 and phase 3 clinical trials that the extent of ongoing transfusional iron load has a marked effect on the ability of deferasirox doses to maintain or reduce hepatic iron.[10,11]
Deferasirox has been reported to be well tolerated in most of the patients, with the most frequent side effects noted being gastrointestinal disturbances, rash, mild non-progressive creatinine increase and elevated liver enzymes. In all above studies, the patients had relatively less iron load than generally seen in Indian patients where cost, compliance and toxicity with DFO and deferiprone have hindered optimal chelation.
The drug was approved by FDA in 2005 and made available in the Indian market in April 2008. It was decided to prospectively study the first 50 patients commencing on this drug at our centre. The present study was conducted with the objectives of evaluating the efficacy and safety of deferasirox in patients with thalassemia major and to monitor the compliance of patients taking deferasirox.
MATERIALS AND METHODS
The first fifty children in the age group of 2-18 years with thalassemia major, to commence deferasirox, were prospectively enrolled in this study in April 2008 and followed up for 36 months till March 2011.
All patients (≥2 year) with thalassemia major and transfusional iron overload (serum ferritin >1000 ng/ml) were eligible for inclusion in the study. All patients were managed according to the standard protocol with the aim of maintaining pre-transfusion hemoglobin levels around 10 gm/dl. Serum ferritin estimation was advised after 10 packed red cell transfusions. Newly diagnosed patients were started on deferasirox if serum ferritin levels reached above 1000 ng/ml. Patients already on chelation were switched to deferasirox if they had unacceptable toxicity, inadequate response, poor compliance or contraindications to other chelators. All patients served as their own historical controls.
Children with age less than 2 years, those with deranged baseline serum creatinine above the upper limit of normal (ULN – 1.2 mg/dl), or patients with elevated baseline liver enzymes (SGOT and SGPT) to more than 4 times the ULN (40 IU/L for both) were excluded from the study.
Baseline serum creatinine, SGOT, SGPT levels and urine albumin were measured along with the mean value of the last three ferritin levels, before starting the therapy. Patients with serum ferritin levels of 1000-1500 ng/ml or requiring less than 14 ml/kg/month of packed red cells were started at an initial dose of 20 mg/kg/day of deferasirox and those with serum ferritin levels of more than 1500 ng/ml or requiring more than 14 ml/kg/month of packed red cells were started at initial dose of 30 mg/kg/day of deferasirox. Dose adjustments were performed based on the serum ferritin trends and if there were adverse effects in steps of 5-10 mg/kg/day to within a range of 20-40 mg/kg/day. Dose was increased if serum ferritin continued to rise on two or more subsequent occasions. The serum creatinine, SGOT, SGPT levels and urine albumin were repeated at monthly intervals for first three months and at three monthly intervals thereafter. The serum ferritin levels were repeated at three monthly intervals.
The drug was discontinued temporarily, if there was derangement in kidney functions, documented by abnormally high serum creatinine values (above ULN), or if the liver enzymes (SGOT and SGPT) rose to more than 5 times the ULN, or if there was a severe skin rash. The drug was restarted, once the adverse event settled.
To assess the compliance, parents were asked to maintain a diary for 100 days of medication taken and calculated the compliance as the number of days the drug was taken out of total 100 days.
Statistical analysis
Data was collected, tabulated and subjected to statistical analysis by using SPSS 17 software. Appropriate statistical tests were applied to various variables.
RESULTS
Data was analyzed for the first 50 patients with thalassemia major aged 2-18 years (mean age 9.62 years) commenced on deferasirox. Majority of the patients were already on alternative chelator (n=44). Three patients were not taking any chelation despite being heavily iron loaded because of poor tolerance. Three patients started deferasirox as their first chelator. The major reason of switching to deferasirox from the previous chelators was noncompliance (n=27, 61%). Eight (18%) patients switched because of rising serum ferritin levels despite optimal doses and compliance to other chelators. Two patients (5%) chose deferasirox because of adverse effects to prior chelation [Table 1].
Table 1
Baseline characteristics of patients
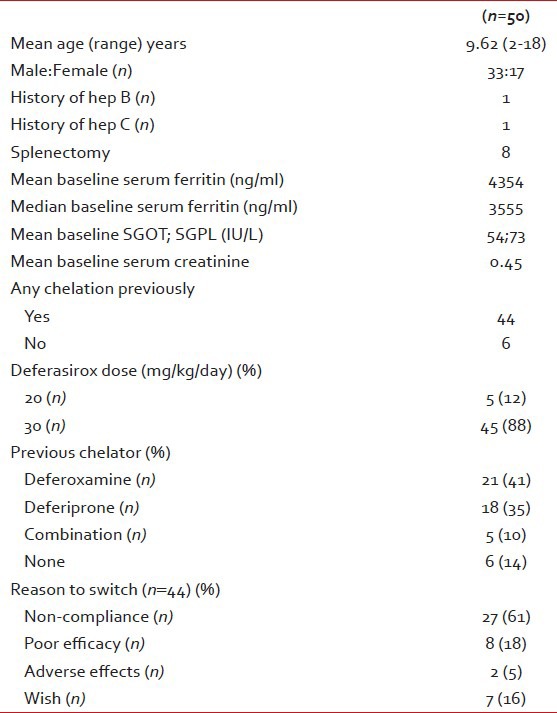
The mean and median serum ferritin of the study group at the start of the study was 4354 ng/ml and 3555 ng/ml, respectively. Eighteen patients (36%) had a serum ferritin of ≥5000 and a majority number had (68%) having a ferritin value of >2500 ng/ml [Figure 1]. The highest ferritin value of this group was 14971 ng/ml. The mean starting dose was 28.89±4.44 mg/kg. Thirty five (70%) patients needed dose increments up to 40 mg/kg/day. The mean deferasirox dose at 36 months was 34.6 mg/kg/day.
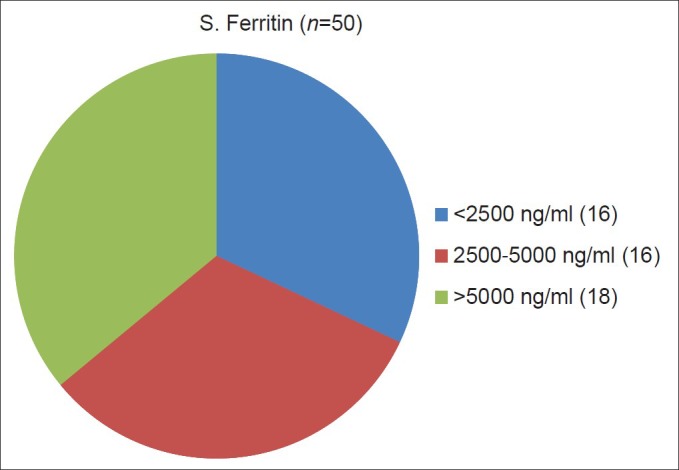
| Fig. 1 Serum ferritin level distribution
The mean SGOT and SGPT were 54 and 73 IU/L, respectively. Ten (19%) and 19 (37%) patients, respectively, had base-line SGOT and SGPT more than twice ULN and 10 (20%) having one or both of these values ≥120 IU/L. Mean serum creatinine of the group was 0.45 mg/dl with four patients (8%) having a serum creatinine value of more than 0.8 mg/dl. None of the patients had serum creatinine more than ULN or significant proteinuria at the baseline.
In the follow-up analysis after 36 months of treatment with deferasirox, majority of patients experienced a fall in serum ferritin levels without any irreversible toxicity. Twenty eight (56%) patients documented a decline in serum ferritin from base-line just after 3 months. However the remaining patients showed an increase but with appropriate dose adjustment up to a maximum of 40 mg/kg/day; at the end of 36 months, 76% showed a significantly lower serum ferritin (decline ≥500) than at baseline. Six patients achieved values lower than 1000 and the drug was temporarily withdrawn in three patients in view of serum ferritin < 500 on two consecutive occasions.
Seven patients have stable serum ferritin (change ≤100). However 5 (10%) patients have continued to show a slow but steady increase despite maximal doses. These patients have been advised to switch back to combination therapy with deferoxamine and deferiprone. However in view of T2 MRI showing stable levels of hemosiderosis, they continue to take deferasirox due to convenience.
The mean ferritin at baseline, 12, 24 and 36 months was 4354, 3260, 3290, 3042, respectively (P=0.003). The median ferritin at the same points was 3555, 2810, 2079, 2271, respectively.
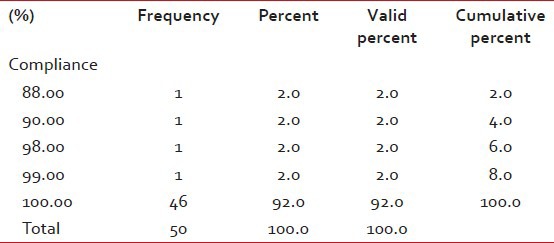
Patients treated with deferasirox showed a definite decrease in mean SGOT, SGPT and serum creatinine values, the absolute numbers being 45, 53 and 0.41, respectively. No patient experienced significant proteinuria during the study period. None of the subjects experienced severe diarrhea, vomiting, skin rash or any other serious adverse effects requiring discontinuation of the drug. All the patients and their parents were satisfied with the new drug, its dosing schedule and its mode of administration. The study group recorded a good compliance of ≥95% [Table 2].
Table 2
Compliance table
In the study group, the mean and median serum ferritin decreased by 1312 and 1284 ng/ml, respectively, over a period of 36 months [Figures [Figures22 and and3].3]. Paired t-test was applied to the initial and final means and a fall in serum ferritin of ≥500 was found to be statistically significant (P=0.003).
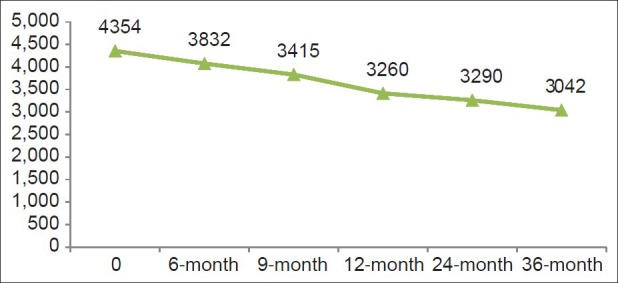
| Fig. 2 Decrease in mean ferritin levels after 36 months of treatment
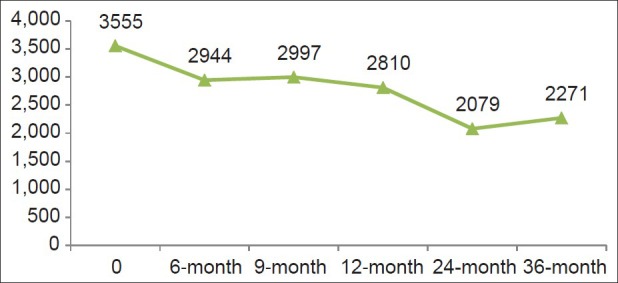
| Fig. 3 Decrease in median ferritin levels after 36 months of treatment
DISCUSSION
Iron overload and transfusional hemosiderosis is the most important determinant of morbidity and mortality in regularly transfused patients with thalassemia major. With the advent of iron chelators such as deferoxamine and deferiprone, the average life span and the quality of life of these patients improved dramatically; however, the mode of administration of deferoxamine and potential serious side effects of deferiprone along with multiple daily doses motivated extensive search for a better chelator, which would be safe and easy to administer apart from being equally or more efficacious. Deferasirox is a designer drug, which was made to combat the above problems and was approved by FDA in 2005 after undergoing extensive safety and efficacy trials in a number of preclinical and clinical settings.
This study was done in our thalassemia unit to evaluate the drug in terms of its effect on serum ferritin, adverse reactions and patient compliance in a population, which was heavily iron loaded, and had some derangement of liver functions at the base-line. Another purpose of this study was to document the efficacy and safety of this drug in an Indian population who due to pharmacogenomic variations may have different pattern of bioavailability and efficacy.
Based on the short-term iron studies and concerns regarding excessive chelation, the initial dose regimens in a number of earlier studies were conservative. The starting dose in this study was higher based on the experience of several investigators that the response of serum ferritin was indeed dose dependent and a minimum dose of 30 mg/kg was needed to achieve a negative iron balance. Cappellini et al.[9] showed in their cohort that at doses of 5-10 mg/kg, the iron stores actually increased while at 20 mg/kg, it was possible to achieve neutral iron balance. However, to achieve a negative iron balance, a minimum of 30 mg/kg was required. Taher et al.[11] also documented this in the seminal escalator study. The safety of deferasirox at doses ≥30 mg/kg has been well documented by Taher et al.[12] The study clearly documented improved iron clearance at doses up to 40 mg/kg/day without any increase in the incidence of adverse events.
The mean starting deferasirox dose used in the study was 28.89±4.44 mg/kg/day, which was based on the premise that a dose of 30 mg/kg/day was required to actively reduce the iron load. The dose was escalated to 40 mg/kg/day in steps of 5 mg/kg/day if there was inadequate response. At the end of 36 months the mean dose was 34.6 mg/kg with 70% of the patients at a dose of 40 mg/kg/day. The serum ferritin of the study group at the start of the study was higher with a majority (67%) having a ferritin value of >2500 ng/ml as compared to earlier studies[5,6,7,9] and by Taher et al.[11] (median baseline ferritin 3396 ng/ml). The mean and median ferritin levels in the present study decreased by 1064 and 1476 ng/ml, respectively. These results were comparable with the findings from the other studies conducted over a period of 12 months, e.g., Cappellini et al.[9] recorded a maximum decrease of 926 ng/ml in mean serum ferritin with a dose of 30 mg/kg/day, and Ali Taher et al.[11] recorded a decrease of 341 ng/ml in median ferritin value.
Pharmacological studies have shown that pharmacokinetics of orally administered deferasirox at doses 10, 20 and 40 mg/kg/day is linear, and drug exposure is dose proportional in patients at steady state.[13] Across the clinical trial program, response to deferasirox was shown to be dependent on dose. Subsequent studies highlighted the additional impact of transfusional iron intake on response.[10,14]
The reasons why a proportion of patients have a dramatic response to this drug whilst others continue to show no response whatsoever remains unclear. All studies have shown that a proportion of patients do not achieve negative iron balance even at 40 mg/kg/day. Pharmacokinetic data has shown that patients who respond poorly have significantly lower systemic exposure compared to responders (P < 0.00001) and that bio-availability is the likely determinant.[13]
The adverse effect profile of the study was almost similar to that seen in other studies, with no serious adverse event attributable to the drug. In the pivotal phase III trial, in which 296 patients with β-thalassemia major received deferasirox for 1 year, the most common adverse events related to deferasirox therapy were gastrointestinal disturbances (15.2%) and rash (10.8%), neither of which required dose adjustment or discontinuation. Increase in serum transaminase above twice the upper limit of normal was reported in 2 of the 296 patients.[5] In the present study, GI disturbances and rash were reported by 10% and 8% of the patients, respectively, all of which were self limited (4-6 days) and did not require any dose modifications. Mild, non-progressive increases in serum creatinine levels were recorded in 53% of the patients but only 11% of these required a temporary discontinuation and none required permanent discontinuation or developed progressive renal dysfunction. Transaminitis were observed in 16% of patients however dose correlations were not observed.
Another significant finding of the study included the improvement of liver enzymes at the end of 24 and 36 months of treatment, which can be attributed to the reduction of the liver iron load. This observation was not seen in earlier studies with deferasirox or with other iron chelators.
Patient satisfaction and drug compliance were excellent and this reflected in the overall efficacy of this drug in reducing iron load. This study reemphasizes the point that drug compliance is very important for a drug to be effective in treating patients with thalassemia major.
CONCLUSIONS
The study results confirm that deferasirox significantly reduces serum ferritin in the majority of patients with thalassemia major. A dose of ≥30 mg/kg is required to achieve a negative iron balance. Thirty five (70%) patients needed dose excalation of up to 40 mg/kg/day. It appears to be well tolerated, safe and efficacious even in patients with very high iron load. There is, however, a subset of patients who fail to achieve negative iron balance despite maximally permissible doses. The optimal strategy for these patients is currently under evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Serum ferritin level distribution

| Fig. 2 Decrease in mean ferritin levels after 36 months of treatment

| Fig. 3 Decrease in median ferritin levels after 36 months of treatment


 PDF
PDF  Views
Views  Share
Share

