Diagnosing Primary Malignancy in Leptomeningeal Carcinomatosis by Using CSF Cytology and Immunohistochemistry: A Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(06): 920-922
DOI: DOI: 10.4103/ijmpo.ijmpo_267_20
Abstract
We present a rare case of recurrent carcinoma of gallbladder with leptomeningeal carcinomatosis. Cerebrospinal fluid (CSF) cytology showed atypical cells, which were suspicious for malignancy on initial reporting. Diagnosis of malignancy and primary from hepatobiliary source was confirmed by doing immunohistochemistry on CSF cell block.
Publication History
Received: 30 May 2020
Accepted: 23 August 2020
Article published online:
14 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We present a rare case of recurrent carcinoma of gallbladder with leptomeningeal carcinomatosis. Cerebrospinal fluid (CSF) cytology showed atypical cells, which were suspicious for malignancy on initial reporting. Diagnosis of malignancy and primary from hepatobiliary source was confirmed by doing immunohistochemistry on CSF cell block.
Introduction
Among the biliary tract malignancies, gallbladder carcinoma (GBC) is the most common worldwide.[1] Compared to southern and western regions, GBC has a higher incidence rate in Northeast and Central India.[2] The incidence rate in North India is 10–12/100,000 population which is similar to high incidence countries of South America. The incidence has been raising steadily, while median survival is less than 6 months and 5-year overall survival is less than 5%.[3] GBC is an aggressive tumor with early dissemination to regional lymph nodes and liver. At the time of diagnosis, 25% of GBCs are localized to the gallbladder wall, 35% have regional lymph nodal involvement, and 45% have distant metastasis. Although systemic metastasis in GBC is common, the central nervous system (CNS) involvement is rare. The incidence of CNS metastasis from GBC is reported as 2%.[4]
We present a case of GBC who received chemotherapy, followed by radical cholecystectomy as with curative intent at another institute. While on follow-up, he presented with leptomeningeal metastasis, which was confirmed by immunohistochemistry (IHC) on cerebrospinal fluid (CSF) cell block.
Case Report
A 61-year-old man with advanced GBC (gallbladder mass infiltrating into segment V and noncontiguous lesion in segment VII of the liver) was treated elsewhere with chemotherapy (6 cycles of gemcitabine and cisplatin from May 2018 to December 2018). In September 2018, after 3 cycles of chemotherapy, positron emission tomography–computed tomography (PET-CT) showed partial response. The patient received 3 more cycles of chemotherapy with gemcitabine and cisplatin. In February 2019, he underwent radical cholecystectomy with excision of hepatic lesion. Histopathology showed well-differentiated adenocarcinoma (residual in the gallbladder) ypT1 N0. Later, he was on maintenance chemotherapy with tablet capecitabine for 6 months.
In the last week of March 2020 (6 months after completing maintenance), he came to the emergency department of our hospital with complaints of difficulty in passing urine and constipation, radiating pain in bilateral lower limbs, difficulty in speaking, and hoarseness of voice which was associated with cough while taking food.
On evaluation, magnetic resonance imaging brain and thoraco-lumbo-sacral spine showed heterogeneous hyperintense lesion [Figure 1] in the parasagittal region and patchy dural enhancement in the cervicodorsal region. Videostroboscopy showed left vocal cord palsy. Fluorodeoxyglucose PET-CT ruled out disease at other sites. These findings pointed to bulbar palsy as a possible cause of his hoarseness of voice and dysphagia. The bulbar palsy was likely a result of leptomeningeal disease. CSF was planned to confirm the same. CSF showed atypical cells, which were suspicious of malignancy, CSF protein was 266.7 mg/dl, and glucose was 29.4 mg/dl. To confirm the diagnosis and primary, IHC markers were done on cell blocks from CSF [Figure 2]. Cell block from CSF showed malignant cell clusters in [Figure 2]a and [Figure 2]b. CK7-positive cells are in [Figure 2]c and MUC-1 (Mucin-1, cell surface associated protein (clone MMAB,BioSB))-positive cells in [Figure 2]d. Cells were negative for CK20 and CDX2. Diagnosis of metastatic adenocarcinoma was confirmed on CSF with strong MUC1 staining favoring pancreatobiliary origin. For dysphagia, nasogastric feeding was started. Intrathecal chemotherapy (IT) was given with methotrexate and hydrocortisone weekly. Post 3 weeks of IT chemotherapy, the patient had symptomatically improved. However, the patient succumbed to chest infection in April 2020.
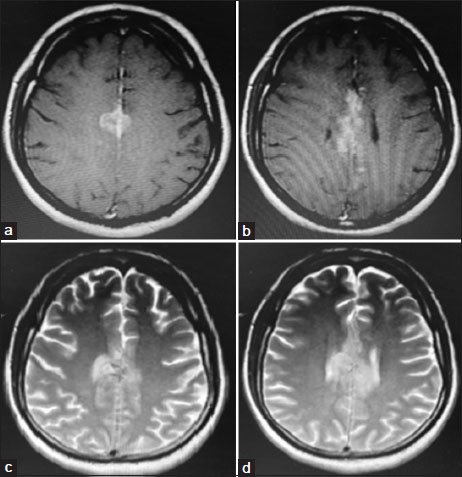
| Figure. 1(a and b) T1-weighted images showing intermediate signal lesions. (c and d) T2-weighted images showing hyperintense lesions
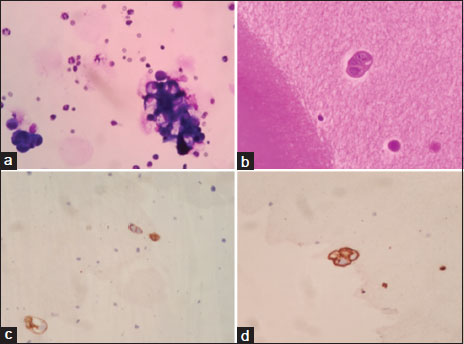
| Figure. 2 (a) Cytocentrifuge smears from cerebrospinal fluid showing few malignant cell clusters in hemorrhagic background (MGG stain, original magnification × 400). (b) Section from cell block reveals only an occasional malignant epithelial cell cluster (H and E stain, original magnification × 400). (c) Immunohistochemistry for CK7 shows positive staining in tumor cells (CK7 immunohistochemistry, original magnification × 400). (d) Immunohistochemistry for MUC1 showing positive staining in tumor cells (MUC1 immunohistochemistry, original magnification × 400)
Discussion
GBC metastasis to CNS is a rare Phenomenon. The most common manifestation of CNS involvement is leptomeningeal carcinomatosis (LMC).[5],[6] Symptoms associated with LMC are headache, paresthesia, cranial nerve palsies, radicular pain, alteration in mental status, and cerebellar dysfunction. A combination of clinical examination, neuroimaging, and lumbar puncture (LP) helps in diagnosing LMC.[7] The presence of malignant cells in CSF is diagnostic. Other features supporting diagnosis are raised CSF pressure, high protein, low glucose, and pleocytosis. In LMC, CSF protein >45 mg/dl is seen in 63%–90% of patients, CSF pressure >150 mm seen in 30%–57% of patients, pleocytosis seen in 33%–79%, and decreased glucose levels (<60 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_267_20#JR_8" xss=removed>8] In our case, CSF showed atypical cells, which were suspicious of malignancy. Delayed CSF smear preparation is known to alter the cytology picture and hence results in ambiguous reporting. Paucicellularity also results in difficult diagnosis. Establishment of diagnosis is critical for management, and repeat lumbar puncture is generally not favored by patients.
CSF analysis by IHC, fluorescent in situ hybridization, polymerase chain reaction, and flow cytometry is known to improve the diagnostic rates. The sensitivity and specificity of IHC in LMC are 0.54 and 0.98, respectively.[7] IHC markers done on cell block from CSF in our case clearly established carcinomatosis (CK positive) and also confirmed hepatobiliary primary (MUC1, CK7 positive and CK 20, CDX2 negative).
There are no clear guidelines for the management of LMC. The treatment is mainly palliative either using IT chemotherapy or radiotherapy. Despite these therapies, the prognosis is guarded and ranges from 3 to 6 months.[9] Various agents have been employed for use in IT chemotherapy, namely methotrexate, cytarabine, and thiotepa.[10]
GBC recurrence with solitary brain metastasis is rare. The case reports of GBC with CNS metastasis with or without other sites of metastasis available in the literature are presented in [Table 1].
|
Case report |
Presentation |
|---|---|
|
Kawamata et al.[11] |
Left cerebellopontine angle tumor with osteolytic changes in left petrous apex mimicking a tentorial meningioma |
|
Takano et al.[12] |
Left frontal cystic lesion. |

| Figure. 1(a and b) T1-weighted images showing intermediate signal lesions. (c and d) T2-weighted images showing hyperintense lesions

| Figure. 2 (a) Cytocentrifuge smears from cerebrospinal fluid showing few malignant cell clusters in hemorrhagic background (MGG stain, original magnification × 400). (b) Section from cell block reveals only an occasional malignant epithelial cell cluster (H and E stain, original magnification × 400). (c) Immunohistochemistry for CK7 shows positive staining in tumor cells (CK7 immunohistochemistry, original magnification × 400). (d) Immunohistochemistry for MUC1 showing positive staining in tumor cells (MUC1 immunohistochemistry, original magnification × 400)
References
- Wistuba II, Gazdar AF. Gallbladder cancer: Lessons from a rare tumour. Nat Rev Cancer 2004; 4: 695-706
- Mhatre SS, Nagrani RT, Budukh A, Chiplunkar S, Badwe R, Patil P. et al. Place of birth and risk of gallbladder cancer in India. Indian J Cancer 2016; 53: 304-8
- Lobo L, Prasad K, Satoskar RR. Carcinoma of the gall bladder. J Clin Diagn Res 2012; 6: 692-5
- Sons HU, Borchard F, Joel BS. Carcinoma of the gallbladder: Autopsy findings in 287 cases and review of the literature. J Surg Oncol 1985; 28: 199-206
- Naylor AR, MacGregor JE, Hutcheon AW, Best PV. Meningeal carcinomatosis from a clinically undiagnosed signet ring cell primary in the gall bladder. Surg Neurol 1988; 29: 315-8
- Miyagui T, Luchemback L, Teixeira GH, de Azevedo KM. Meningeal carcinomatosis as the initial manifestation of a gallbladder adenocarcinoma associated with a Krukenberg tumor. Rev Hosp Clin Fac Med Sao Paulo 2003; 58: 169-72
- Hovestadt A, Henzen-Logmans SC, Vecht CJ. Immunohistochemical analysis of the cerebrospinal fluid for carcinomatous and lymphomatous leptomeningitis. Br J Cancer 1990; 62: 653-4
- Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol 2006; 5: 443-52
- Mack F, Baumert BG, Schäfer N, Hattingen E, Scheffler B, Herrlinger U. et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev 2016; 43: 83-91
- 10 Grossman SA, Krabak MJ. “Leptomeningeal carcinomatosis. ” Cancer Treat Rev 1999; 25: 103-19
- 11 Kawamata T, Kawamura H, Kubo O, Sasahara A, Yamazato M, Hori T, Sarin YK. “Central nervous system metastasis from gallbladder carcinoma mimicking a meningioma. Case illustration.”. J Neurosur 1999; 91: 1059
- Takano S, Yoshii Y, Owada T, Shirai S, Nose T. et al. ”Central nervous system metastasis from gallbladder carcinoma. Case report.”. Neurologia Medico-Chirurgica 1991; 31: 782-6
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int J Cancer 2006; 118: 1591-602


 PDF
PDF  Views
Views  Share
Share

