Down-staging following neoadjuvant chemo-radiotherapy for locally advanced rectal cancer: Does timing of surgery really matter
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(04): 263-266
DOI: DOI: 10.4103/0971-5851.144986
Abstract
Background: Neoadjuvant chemoradiotherapy (NACTRT) improves local recurrence rate in locally advanced (LA) rectal cancer with no survival benefit. Pathological complete response (pCR) post-NACTRT is associated with improved outcome. Debate is ongoing as to when would be the opportune time to operate. Aim: To determine if greater down-staging can be achieved by a longer time interval from NACTRT to surgery (tumor regression score [TRS]) and whether this would impact sphincter saving surgery rates and early relapse rates. Materials and Methods: A retrospective analysis of a prospectively maintained database of patients with LA rectal adenocarcinoma treated from January 2012 to August 2013 was carried out. One hundred and ten patients who completed NACTRT (50 Gy/25 fractions with capecitabine 825 mg/m 2 twice daily) followed by surgical resection were included. For response evaluation patients were divided into two groups, Group 1 (TRS ≤60 days, n = 42) and 2 (TRS >60 days, n = 68). Tumor down-staging, pCR rate, tumor regression grade (TRG) post-NACTRT and relapse rates were correlated with TRS. Results: Of 110 patients (median age: 49 years (21-73), 71% males; 18 (16.5%) with signet ring histology) 96% patients underwent an R0 resection. Post-NACTRT, CR was attained in 5 (4.5%), partial response in 98 (89%) and stable disease in 7 (6.4%) patients. Median time from completion of NACTRT to surgery was 64.5 days (6-474). Median lymph nodes harvested were 10 (1-50). Overall, 22 (20%) patients achieved pCR. 26 (62%) patients in Group 1 compared to 36 (53%) in Group 2 underwent sphincter sparing surgery (SSS) (P = 0.357). Six patients (14%) in Group 1 and 16 (24%) in Group 2 achieved pCR (P = 0.24). Median TRG in both groups was three. Conclusion: Timing of surgery following NACTRT for LA rectal cancer does not influence pathological response, ability to perform SSS or disease-free survival. There is no incremental benefit of delaying the surgery though this needs to be confirmed in a prospective randomized trial.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Neoadjuvant chemoradiotherapy (NACTRT) improves local recurrence rate in locally advanced (LA) rectal cancer with no survival benefit. Pathological complete response (pCR) post-NACTRT is associated with improved outcome. Debate is ongoing as to when would be the opportune time to operate.
Aim:
To determine if greater down-staging can be achieved by a longer time interval from NACTRT to surgery (tumor regression score [TRS]) and whether this would impact sphincter saving surgery rates and early relapse rates.
Materials and Methods:
A retrospective analysis of a prospectively maintained database of patients with LA rectal adenocarcinoma treated from January 2012 to August 2013 was carried out. One hundred and ten patients who completed NACTRT (50 Gy/25 fractions with capecitabine 825 mg/m2 twice daily) followed by surgical resection were included. For response evaluation patients were divided into two groups, Group 1 (TRS ≤60 days, n = 42) and 2 (TRS >60 days, n = 68). Tumor down-staging, pCR rate, tumor regression grade (TRG) post-NACTRT and relapse rates were correlated with TRS.
Results:
Of 110 patients (median age: 49 years (21-73), 71% males; 18 (16.5%) with signet ring histology) 96% patients underwent an R0 resection. Post-NACTRT, CR was attained in 5 (4.5%), partial response in 98 (89%) and stable disease in 7 (6.4%) patients. Median time from completion of NACTRT to surgery was 64.5 days (6-474). Median lymph nodes harvested were 10 (1-50). Overall, 22 (20%) patients achieved pCR. 26 (62%) patients in Group 1 compared to 36 (53%) in Group 2 underwent sphincter sparing surgery (SSS) (P = 0.357). Six patients (14%) in Group 1 and 16 (24%) in Group 2 achieved pCR (P = 0.24). Median TRG in both groups was three.
Conclusion:
Timing of surgery following NACTRT for LA rectal cancer does not influence pathological response, ability to perform SSS or disease-free survival. There is no incremental benefit of delaying the surgery though this needs to be confirmed in a prospective randomized trial.
INTRODUCTION
Neoadjuvant chemoradiation (NACTRT) has been widely used in the treatment of patients with locally advanced (LA) rectal cancer owing to its ability to reduce local recurrence rates[1,2,3,4] as well as influence survival (in some early randomized controlled trials).[1,2] NACTRT results in tumor down-staging and regression, facilitating resection in those with threatened circumferential resection margin (CRM) as well as allows for the performance of sphincter sparing surgery (SSS).[4,5]
However, in recent times, the focus has shifted toward determining the opportune time to offer surgery following completion of NACTRT (tumor regression score [TRS]).[6,7,8] Radiation therapy has been shown to have an ongoing effect on tumor necrosis for weeks beyond completion of NACTRT.[8] This correlates clinically with increased tumor as well as nodal down-staging and increased pathological complete response (pCR) rates associated with TRS of more than 6-7 weeks.[9,10,11,12] Besides, the trend toward reduced complication rates was noted by Jeong et al.[11] Interestingly, this has not uniformly translated to clinically relevant end-points of increased rates of SSS, nor the survival.[9,10] Thus, to this day, it is unclear if prolonging TRS to more than 8 weeks would be of any clinical benefit.[6]
The aim of the current study was thus to compare the benefits, if any, of increasing TRS to more than 8 weeks (60 days) versus <8>
MATERIALS AND METHODS
An analysis of patients with LA rectal cancer from a prospectively maintained database of all colorectal cancer patients treated surgically at the tertiary cancer center from January 2012 to August 2013 was performed. The objectives were to determine if greater down-staging can be achieved by a longer time interval from NACTRT to surgery (TRS) and whether this would impact SSS rates and early relapse rates.
Rectal cancer was defined as a cancer arising or lower margin extending to within 15 cm of the anal verge. Preoperatively, all patients were investigated as follows:[13]
- Complete colonoscopy (up to the caecum; in case of constricting tumors in which a complete colonoscopy was not possible, a proctosopcic biopsy was obtained, and the patient was advised a colonoscopy within 1-year of the primary diagnosis),
- Blood investigations — including blood counts, liver and renal functions,
- Serum tumor marker — carcinoembryonic antigen,
- Imaging - magnetic resonance imaging of pelvis and computed tomography scan of chest and abdomen.
Inclusion criteria
Patients aged more than 18 years with histologically proven adenocarcinoma of the rectum who received NACTRT were included. Indications for NACTRT included nonmetastatic disease with threatened CRM, tumor abutting inter-sphincteric plane, T3-4 disease, extramural vascular invasion and lymph node positivity on imaging.
Exclusion criteria
Patients who could not complete NACTRT, had disease progression, while on NACTRT and those who did not undergo surgical resection were excluded.
Study methodology
The flow chart in Figure 1 shows the methodology for this study. TRS was calculated from the day of completion of RT to the day of surgery. Delay in surgery beyond 60 days was either the result of a long waiting list for surgery or due to the wish of the patient to delay surgery owing to personal factors.
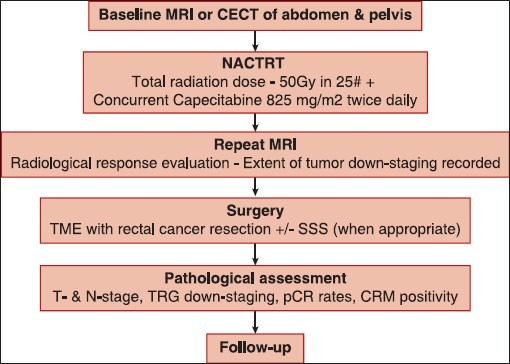
| Figure 1:Flow chart depicting the conduct of the study. A baseline computed tomography was used in some patients who did not have baseline magnetic resonance imaging (MRI – Magnetic resonance imaging; CECT – Contrast-enhanced computed tomography; pCR – Pathological complete response; TME – Total mesorectal excision; SSS – Sphincter saving surgery; CRM – Circumferential resection margin; T – Tumor; N – Node)
The data were collected in the course of common clinical practice and accordingly, the signed informed consent was obtained from each patient for any surgical and clinical procedure.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, IBM Inc.) Version 18.0 for Windows. Nominal data is provided as number (%) and continuous data as median (range). One hundred and twelve patients who completed NACTRT followed by surgical resection were included. For the analysis, patients were divided into two groups, Group 1 (TRS ≤60 days, n = 42) and 2 (TRS >60 days, n = 68) to evaluate the impact of TRS >8 weeks on clinical end-points. Tumor down-staging, pCR rate, TRG post-NACTRT and relapse rates were correlated with TRS. The association for categorical variables between groups stratified by TRS was assessed using Fisher's exact test. Means were compared using Student's t-test. P < 0>
RESULTS
Patient characteristics
Seventy-eight of the 110 patients (71%) were males with a median age of 49 years (range: 21-73). 18 patients (16.5%) had signet ring histology in the entire cohort. Radiological response to NACTRT was complete response (CR) in 5 (4.5%), partial response in 98 (89%) and stable disease in 7 (6.4%) patients. Median time interval from completion of NACTRT to surgery (TRS) was 64.5 days (range: 6-474), and 96% patients underwent a complete (R0) resection.
Table 1 provides a comparison of the basic patient-related factors between the two groups. The only significant difference between the groups was in terms of the distance of the lower edge of the tumor from the anal verge (median [cm]: 6 vs. 4; P = 0.045).
Table 1
Comparison of patient-related factors between the two groups
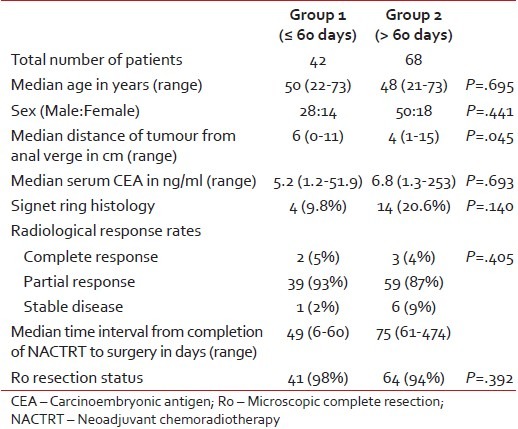
|
Pathological factors
Overall, 22 (20%) patients achieved pCR, while 5 (4.5%) patients had a positive CRM. A median of 10 lymph nodes (range: 1-50) were resected. Table 2 provides a comparison of the pathological factors between the two groups.
Table 2
Comparison in the pathological variables between the two groups
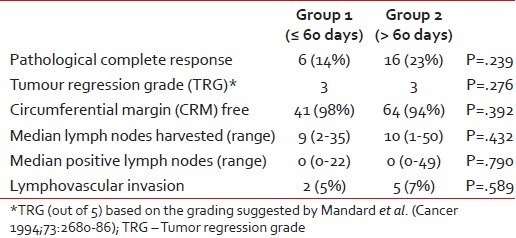
|
Follow-up
Over a median follow-up of 13 months (range: 4-27), a total of 20 patients (18%) developed recurrences - 6 in Group 1 (14%) and 14 (21%) in Group 2 (P = 0.405). Table 3 provides a comparison between the two groups in terms of the location of recurrence.
Table 3
Comparison in the sites of recurrence between the two groups
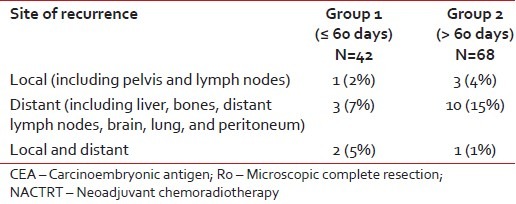
|
DISCUSSION
These data suggest that there exist no differences in the pathological response rates, the ability to perform SSS or the early disease-free survival between patients operated within 60 days (8 weeks) following completion of NACTRT when compared to those operated after 60 days.
Pathological CR is generally regarded as a surrogate marker for disease-free survival.[14] In the absence of a clear lack of benefit of NACTRT on overall survival,[3,4] the focus in rectal cancer is centered on delaying disease-free survival. Based on some studies,[12,15,16] it was felt that delaying surgery may help in achieving better pCR rates and hence, improved disease-free survival. A recent meta-analysis,[17] too, has suggested that delaying surgery to beyond 6-8 weeks may help improve pCR rates by 6%. However, there exists a lot of heterogeneity between the studies to draw firm conclusions.[6] In fact in the meta-analysis by Petrelli et al.,[17] there were only three studies that actually compared surgery before and after 8 weeks of chemoradiotherapy.[18,19,20] The study by Tran et al.[18] solely dealt with safety of delaying surgery beyond 8 weeks. While de Campos-Lobato et al.[19] found a higher rate of pathologic CR and decreased local recurrence, Stein et al.[20] found no significant difference in neither tumor or nodal down-staging, nor the pathological response. In our study, we have drawn similar inferences to Stein et al.[20] In addition, we have been able to note the lack of difference in disease-free survival.
The other proposed benefit of delaying surgery after NACTRT was it's supposed propensity to result in higher sphincter preservation.[8] However, as noted in other studies,[10,20,21] we did not find any significant difference in sphincter preservation rates as a result of delaying surgery beyond 60 days. Even though patients in whom surgery was delayed beyond 60 days had lower rectal tumors, there was no difference in the two groups.
The treatment of esophageal cancer, like rectal cancer, has seen a paradigm shift from upfront surgery to NACTRT in the last decade. However, there too, there has been no real benefit noted from delaying surgery beyond the 8 weeks mark[22,23] which leads us to infer that the current lack of clarity in the literature on deciding the optimal timing for surgery following NACTRT has possibly arisen owing to the reliance on solely pCR as a surrogate marker for disease-free survival.[14] Maybe, in addition to pathological factors, it is time to explore genetic or immune factors[24] that may guide us in making the right decision not only on timing of surgery, but other treatment modalities, as well, that we may aid in improving the overall outcome and survival in patients with rectal cancer.
CONCLUSION
Timing of surgery (≤60 days vs. >60 days) following NACTRT for LA rectal cancer does not influence either pathological response, the ability to perform sphincter-saving procedures or the early disease-free survival. Hence, based on these data it is safe to say that surgery may be delayed in some patients if logistics so dictate though there is no incremental benefit of delaying the surgery. This needs to be confirmed in a prospective randomized trial.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Kodner IJ, Shemesh EI, Fry RD, Walz BJ, Myerson R, Fleshman JW, et al. Preoperative irradiation for rectal cancer. Improved local control and long-term survival. Ann Surg 1989;209:194-9.
- Randomized study on preoperative radiotherapy in rectal carcinoma. Stockholm Colorectal Cancer Study Group. Ann Surg Oncol 1996;3:423-30.
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33.
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40.
- Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: Long term follow-up. Int J Radiat Oncol Biol Phys 1998;42:51-7.
- Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: A systematic review of the literature. Dis Colon Rectum 2013;56:921-30.
- Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 2008; 71:1181-8.
- Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on down-staging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396.
- Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7.
- ;Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004;47:279-86.
- Jeong DH, Lee HB, Hur H, Min BS, Baik SH, Kim NK. Optimal timing of surgery after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Korean Surg Soc 2013;84:338-45.
- Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933-9.
- Barreto SG, Chaubal GN, Talole S, DeSouza A, Suradkar K, Gaikwad V, et al. Rectal cancer in young Indians - Are these cancers different compared to their older counterparts? Indian J Gastroenterol 2014;33:146-50.
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44.
- Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833-41.
- Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102.
- Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: A meta-analysis of published studies. Ann Surg 2013.
- Tran CL, Udani S, Holt A, Arnell T, Kumar R, Stamos MJ. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006;192:873-7.
- de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: The impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444-50.
- Stein DE, Mahmoud NN, Anné PR, Rose DG, Isenberg GA, Goldstein SD, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve down-staging of rectal carcinoma. Dis Colon Rectum 2003;46:448-53.
- Lim SB, Choi HS, Jeong SY, Kim DY, Jung KH, Hong YS, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008;248:243-51.
- Chiu CH, Chao YK, Chang HK, Tseng CK, Chan SC, Liu YH, et al. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: Does delayed surgery impact outcome? Ann Surg Oncol 2013;20:4245-51.
- Tessier W, Gronnier C, Messager M, Hec F, Mirabel X, Robb WB, et al. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg 2014;97:1181-9.
- Sermeus A, Leonard W, Engels B, De Ridder M. Advances in radiotherapy and targeted therapies for rectal cancer. World J Gastroenterol 2014;20:1-5.

| Figure 1:Flow chart depicting the conduct of the study. A baseline computed tomography was used in some patients who did not have baseline magnetic resonance imaging (MRI – Magnetic resonance imaging; CECT – Contrast-enhanced computed tomography; pCR – Pathological complete response; TME – Total mesorectal excision; SSS – Sphincter saving surgery; CRM – Circumferential resection margin; T – Tumor; N – Node)
References
- Kodner IJ, Shemesh EI, Fry RD, Walz BJ, Myerson R, Fleshman JW, et al. Preoperative irradiation for rectal cancer. Improved local control and long-term survival. Ann Surg 1989;209:194-9.
- Randomized study on preoperative radiotherapy in rectal carcinoma. Stockholm Colorectal Cancer Study Group. Ann Surg Oncol 1996;3:423-30.
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33.
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40.
- Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: Long term follow-up. Int J Radiat Oncol Biol Phys 1998;42:51-7.
- Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: A systematic review of the literature. Dis Colon Rectum 2013;56:921-30.
- Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 2008; 71:1181-8.
- Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on down-staging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396.
- Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7.
- ;Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004;47:279-86.
- Jeong DH, Lee HB, Hur H, Min BS, Baik SH, Kim NK. Optimal timing of surgery after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Korean Surg Soc 2013;84:338-45.
- Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933-9.
- Barreto SG, Chaubal GN, Talole S, DeSouza A, Suradkar K, Gaikwad V, et al. Rectal cancer in young Indians - Are these cancers different compared to their older counterparts? Indian J Gastroenterol 2014;33:146-50.
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44.
- Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833-41.
- Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102.
- Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: A meta-analysis of published studies. Ann Surg 2013.
- Tran CL, Udani S, Holt A, Arnell T, Kumar R, Stamos MJ. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006;192:873-7.
- de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: The impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444-50.
- Stein DE, Mahmoud NN, Anné PR, Rose DG, Isenberg GA, Goldstein SD, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve down-staging of rectal carcinoma. Dis Colon Rectum 2003;46:448-53.
- Lim SB, Choi HS, Jeong SY, Kim DY, Jung KH, Hong YS, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008;248:243-51.
- Chiu CH, Chao YK, Chang HK, Tseng CK, Chan SC, Liu YH, et al. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: Does delayed surgery impact outcome? Ann Surg Oncol 2013;20:4245-51.
- Tessier W, Gronnier C, Messager M, Hec F, Mirabel X, Robb WB, et al. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg 2014;97:1181-9.
- Sermeus A, Leonard W, Engels B, De Ridder M. Advances in radiotherapy and targeted therapies for rectal cancer. World J Gastroenterol 2014;20:1-5.


 PDF
PDF  Views
Views  Share
Share

