Epidemiologic Study of Patients Registered in Oncology Unit at a Hepatobiliary Tertiary Care Center in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 358-367
DOI: DOI: 10.4103/ijmpo.ijmpo_196_18
Abstract
Background: Cancer accounts for about 1 in 7 deaths, worldwide. Primary cancers of the hepatobiliary system are significant health problems worldwide and their management presents great challenges for the hepatobiliary specialist. The incidence of hepatobiliary malignancies is on an increasing trend in India. Study: We did a retrospective study for the epidemiologic, clinical characteristics, and outcomes of patients with cancer registering for treatment in the oncology division at the Institute of Liver and Biliary Sciences, Delhi, India, between January 1, 2017 and December 31, 2017. Results: Atotal of 502 new patients were registered during the study period. The majority of the patients were male (M:F 1.69:1), in the age group of 35–64 years (64.3%) and presented in advanced stages of the disease (72.7% in Stage III and IV). The most common cancers were gallbladder cancer (GBC) (29.7%) and hepatocellular carcinoma (HCC) (17.3%). GBC was the most common in females (M: F 1:1.6), 86.6% were advanced (Stage III and IV), and gallstones were present in 44.3% patients (M: F 1:2.9). Periampullary carcinoma presented in early stages (71% in Stage I and II). Survival at 6 months (n = 110 evaluable patients) was 100% for Stage I, 88% for Stage II, 73.7% for Stage III and 42.1% for Stage IV, and 62.7% overall (P < 0.001). Survival at 6 months (n = 123 evaluable patients) was 56.5% for biliary cancers, 71.4% for HCC, and 75% for nonbiliary cancers (P = 0.15). 217 (43%) patients had one visit to the hospital and 168 (34%) patients had 2–5 visits with no or little follow-up. Conclusions: Most of the disease burden was in the male gender (GBC was more common in females), in the age group 35–64 years and with advanced disease presentation (except periampullary cancer). Survival diminished significantly with increasing stage of disease. Survival was worse for patients with biliary cancers. This could be due to advanced presentation, poor follow-up, and inadequate public health awareness.
Keywords
Epidemiology - gallbladder cancer - hepatobiliary cancers - hepatocellular cancer - periampullary carcinomaPublication History
Received: 07 September 2018
Accepted: 10 November 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Cancer accounts for about 1 in 7 deaths, worldwide. Primary cancers of the hepatobiliary system are significant health problems worldwide and their management presents great challenges for the hepatobiliary specialist. The incidence of hepatobiliary malignancies is on an increasing trend in India. Study: We did a retrospective study for the epidemiologic, clinical characteristics, and outcomes of patients with cancer registering for treatment in the oncology division at the Institute of Liver and Biliary Sciences, Delhi, India, between January 1, 2017 and December 31, 2017. Results: Atotal of 502 new patients were registered during the study period. The majority of the patients were male (M:F 1.69:1), in the age group of 35–64 years (64.3%) and presented in advanced stages of the disease (72.7% in Stage III and IV). The most common cancers were gallbladder cancer (GBC) (29.7%) and hepatocellular carcinoma (HCC) (17.3%). GBC was the most common in females (M: F 1:1.6), 86.6% were advanced (Stage III and IV), and gallstones were present in 44.3% patients (M: F 1:2.9). Periampullary carcinoma presented in early stages (71% in Stage I and II). Survival at 6 months (n = 110 evaluable patients) was 100% for Stage I, 88% for Stage II, 73.7% for Stage III and 42.1% for Stage IV, and 62.7% overall (P < 0.001). Survival at 6 months (n = 123 evaluable patients) was 56.5% for biliary cancers, 71.4% for HCC, and 75% for nonbiliary cancers (P = 0.15). 217 (43%) patients had one visit to the hospital and 168 (34%) patients had 2–5 visits with no or little follow-up. Conclusions: Most of the disease burden was in the male gender (GBC was more common in females), in the age group 35–64 years and with advanced disease presentation (except periampullary cancer). Survival diminished significantly with increasing stage of disease. Survival was worse for patients with biliary cancers. This could be due to advanced presentation, poor follow-up, and inadequate public health awareness.
Keywords
Epidemiology - gallbladder cancer - hepatobiliary cancers - hepatocellular cancer - periampullary carcinomaIntroduction
Cancer is an enormous global health burden. Today, cancer accounts for about 1 in 7 deaths, worldwide – more than HIV/AIDS, tuberculosis, and malaria combined. In 2012, an estimated 14.1 million cases of cancer were diagnosed the worldwide and 8.2 million cancer deaths.[1] In 2030, about 21.6 million new cancer cases and 13 million cancer deaths are expected to occur.[1] India is likely to have over 17.3 lakh new cases of cancer and over 8.8 lakh cancer deaths by 2020 with cancers of the breast, lung, and cervix topping the list.[2]
Primary cancers of the hepatobiliary system are significant health problems worldwide and their management presents great challenges for the hepatobiliary specialist. The biliary tract includes the gallbladder and the intra- and extra-hepatic bile ducts. Cancers of the biliary tract may be associated with biliary tract epithelia along the entire biliary tract from the intrahepatic ductules to the ampulla of Vater. They include gallbladder cancer (GBC), cholangiocarcinoma (CCA), and periampullary cancers.
Liver cancer is the fifth-most common cancer in men worldwide (7.5%) and ninth in women (3.4%).[3] It is largely a problem of the less developed regions where 83% (50% in China alone) of the estimated 782,000 new cancer cases worldwide occurred in 2012. This is due to the relationship of hepatocellular carcinoma (HCC) to chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections.[4] It is the third-most common cause of death from cancer worldwide, estimated to be responsible for nearly 781,631 deaths in 2018 (8.2%).[3] The prognosis for liver cancer is very poor (overall ratio of mortality to incidence is 0.95). In India, liver cancer is the ninth-most common cancer in men (Age Standardized Rate
In most countries, HCC accounts for 70%–85% of primary liver cancer cases.[5] with the burden of disease expected to increase in the coming years.[6] The combination of cancer and chronic liver disease adds significant complexity to treatment. The variable geographic incidence of liver cancer reflects the variable geographic incidence in HCV and HBV infections, which account for 75% of the world's cases.[7]
GBC is the fifth-most common malignancy of the gastrointestinal tract (GIT). GBC affects women three to four times more often than men.[1] In India, GBC is the twelfth-most common cancer in men (ASR incidence 1.5 and ASR mortality 1.3) and eleventh in women (ASR incidence 2.1 and ASR mortality 1.8.[3] The incidence of GBC correlates with the prevalence of cholelithiasis (gallstones). Patients who have had gallstones for longer than 40 years have a significantly higher incidence of GBC than those who have had gallstones for a shorter time.
Institute of Liver and Biliary Sciences (ILBS), Delhi is a one of its kind institution in India which provides comprehensive care for patients with hepatobiliary diseases including cancers. We are in a unique position for conducting research in these malignancies, some of which are rare cancers, and most of them have a dismal prognosis. The institute commonly deals with liver cancers (primary and metastatic), biliary tract cancers along with pancreatic, neuroendocrine tumors (NETs), and other GIT cancers.
In this retrospective study, we aimed to study the epidemiologic, clinical characteristics, and outcomes of patients with cancer registering for treatment in the oncology division of ILBS. We planned this study to increase our understanding of these cancers and deliver improved care to patients.
Materials and Methods
The study was conducted in the oncology division of ILBS, Delhi and included all new patients who were registered from January 1, 2017 to December 31, 2017.
Patient characteristics with regard to demographic data – age, gender, state of residence, and clinical data – investigations, diagnosis, and stage of disease, were studied in detail. Clinical and laboratory features, as well as radiological imaging, were analyzed. Diagnosis and staging had been confirmed by computed tomography or magnetic resonance imaging scans. Outcomes were analyzed at 6 months from the completion of the study period. Patients with incomplete data were not included in the final analyses. Standard statistical methods were used for statistical analyses.
Results
A total of 502 new patients were registered in the oncology division at ILBS, Delhi, from January 1, 2017 to December 31, 2017. The demographic characteristics of the patients are shown in [Table 1]. The majority of the patients were male (M:F ratio was 1.69:1) [Figure 1] and in the 35–64 age group of years (64.3%) [Figure 2]. Six percent were in the 15–34 years age group and 30% in the 65+ age group. Most of the patients hailed from the states of Delhi (31.5%) and Uttar Pradesh (24.1%).
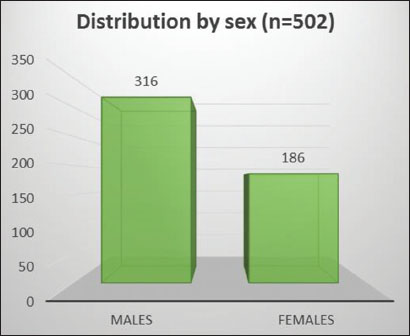
| Figure 1: Distribution of all cancers by sex
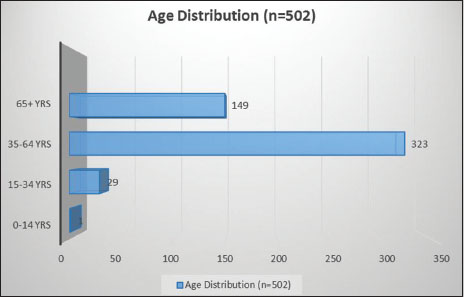
| Figure 2: Distribution of all cancers by age
Table 1Demographic distribution of registered patients (n=502)
|
Characteristics |
Number of patients, n (%) |
|---|---|
|
Sex |
|
|
Male |
316 (62.9) |
|
Female |
186 (37.1) |
|
Age (years) |
|
|
0-14 |
1 (0.2) |
|
15-34 |
29 (5.8) |
|
35-64 |
323 (64.3) |
|
>65 |
149 (29.7) |
|
State of residence |
|
|
Delhi |
158 (31.5) |
|
Uttar Pradesh |
121 (24.1) |
|
Bihar |
47 (9.4) |
|
Haryana |
38 (7.6) |
|
Rajasthan |
20 (4) |
|
Madhya Pradesh |
18 (3.9) |
|
Uttarakhand, Punjab |
12 each (2.4) |
|
Jharkhand |
9 (1.8) |
|
Jammu and Kashmir |
7(1.4) |
|
Assam |
6 (1.2) |
|
Manipur |
5 (1) |
|
West Bengal |
4 (0.8) |
|
Sikkim, Maharashtra |
3 each (0.6) |
|
Orissa, Chhattisgarh, Himachal |
2 each (0.4) |
|
Pradesh, Arunachal Pradesh |
|
|
Meghalaya, Tripura, Gujarat, |
1 each (0.2) |
|
Andhra Pradesh, Karnataka |
|
|
Nepal (country) |
1 (0.2) |
|
Insufficient data of address |
25 (5) |
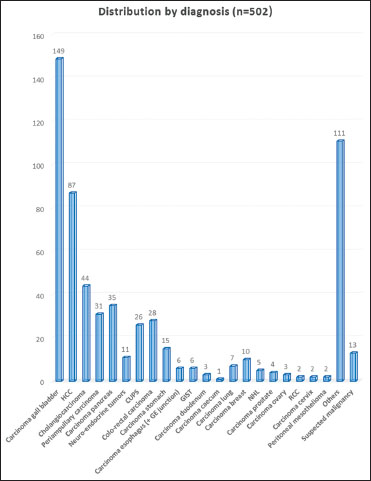
| Figure 3: Distribution of all cancers by diagnosis
Table 2 Distribution by diagnosis of registered patients (n=502)
Majority of the patients presented in advanced stages [Figure 4], 61.7% were Stage IV (American Joint Committee on Cancer TNM staging system - AJCC 2010) and 11% were Stage III. Of the remaining, 10.6% were Stage II, 3.9% were Stage I, and only one patient (0.24%) was Stage 0. Stage was undetermined in 39 patients (9.4%) due to insufficient data.
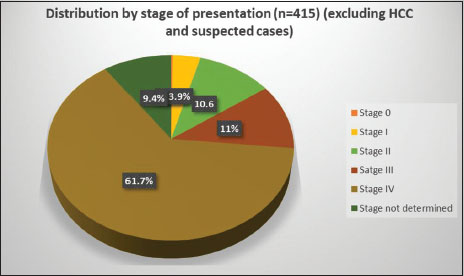
| Figure 4: Distribution of all cancers by stage at presentation (American Joint Committee on Cancer 2010 TNM staging)
Patients with HCC presented in advanced stages [Figure 5], 65.5% were Barcelona Clinic Liver Cancer (BCLC) C (BCLC staging system) and 4.6% were BCLC D. Of the operable cases, 11.5% were BCLC B and 8% were BCLC A. Stage was undetermined in 9 patients (10.3%) due to insufficient data. Most of the patients were male (88.5%) and M:F ratio was 7.7:1.
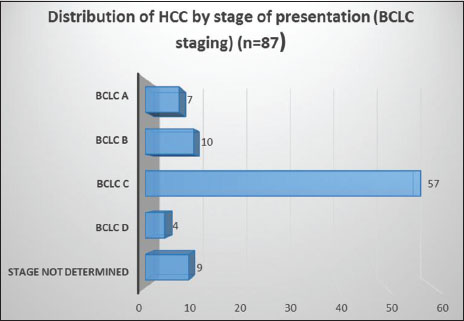
| Figure 5: Distribution of hepatocellular carcinoma by stage at presentation (Barcelona Clinic Liver Cancer staging)
Patients with GBC presented in advanced stages [Figure 6], 75.2% were Stage IV and 11.4% were Stage III. Among the operable cases, 4% were Stage II and 1.34% were Stage I. Stage was undetermined in 12 patients (8%) due to insufficient data. Majority of the patients were female (M:F ratio was 1:1.6) [Figure 7]. Most of the patients were in the 35–64 years' age group (68.5%), 4% in 15–34 years' age group and 27.5% in 65+ years' age group. Gallstones were present at diagnosis in 66 patients (44.3%), of which majority were female, (M:F ratio was 1:2.9) [Figure 7]. Data were insufficient in 11 patients (7.4%). Sludge in gall bladder was seen in seven patients without gallstones.
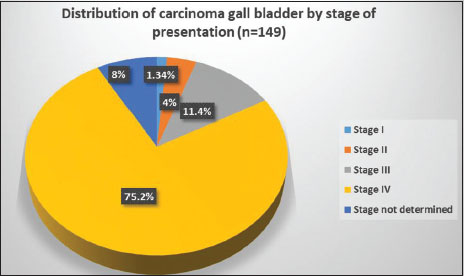
| Figure 6: Distribution of carcinoma gall bladder by stage at presentation (American Joint Committee on Cancer 2010 staging)
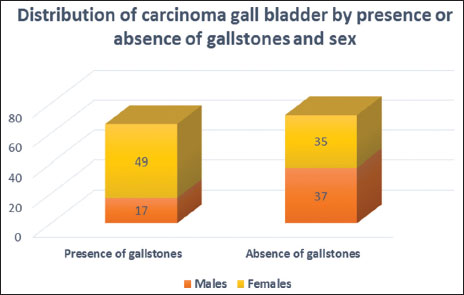
| Figure 7: Distribution of carcinoma gall bladder by the presence or absence of gallstones and sex
Patients with CCA presented in advanced stages [Figure 8] with 59% in Stage IV and 9% in Stage III. Only 4.5% patients were Stage I and 15.9% were Stage II. Stage was undetermined in 5 (11.4%) patients due to insufficient data.
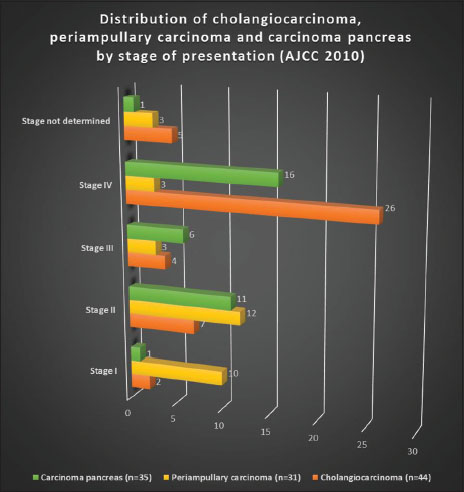
| Figure 8: Distribution of cholangiocarcinoma, periampullary carcinoma, and carcinoma pancreas by stage of presentation (American Joint Committee on Cancer 2010 staging
Patients with periampullary carcinoma had a different pattern of presentation [Figure 8] with 32.3% in Stage I, 38.7% in Stage II, 9.7% in Stage III, and 9.7% in Stage IV disease. Stage was undetermined in 3 (9.7%) patients due to insufficient data.
Patients with carcinoma pancreas presented in advanced stages [Figure 8] with 45.7% Stage IV and 17.1% Stage III. Only 1 (2.9%) patient had Stage I and 31.4% patients had Stage II. Stage was undetermined in 1 (2.9%) patient due to insufficient data.
Distribution of patients with NET showed that 90.9% patients had Stage IV disease. Stage was undetermined in 1 (9.1%) patient due to insufficient data. Further, 27.3% patients had Grade 1, 36.4% patients had Grade 2, and 27.3% patients had Grade 3 disease. Grade was undetermined in 1 (9.1%) patient due to insufficient data. Moreover, 18.2% patients had pancreas as primary site, 54.5% patients had GIT as the primary site, and 27.3% patients had unknown primary.
Outcomes were analyzed at 6 months from the completion of the study. Data were available for 110 patients [Table 3]. Survival differed significantly according to stage of patients [Figure 9]. Overall survival at 6 months was 100% for Stage I patients, 88% for Stage II, 73.7% for Stage III, 42.1% for Stage IV, and 62.7% patients overall. The decline in survival with increasing stage was statistically significant (P < 0.001).
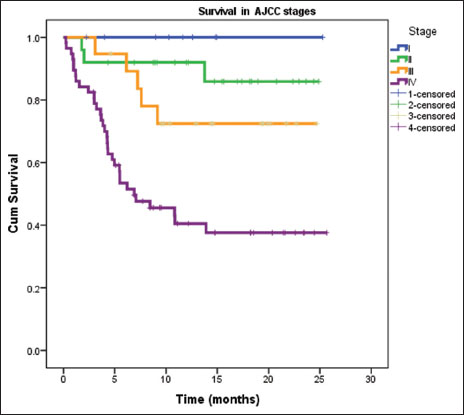
| Figure 9: Kaplan–Meier survival curve of patients at 6 months according to the American Joint Committee on Cancer Stage (n = 110) (P < 0.001)
Survival of patients also differed according to the separation of diagnosis into biliary and nonbiliary cancers [Table 4]. Data were available for 123 patients [Figure 10]. Overall survival at 6 months was 56.5% for biliary cancers, 71.4% for HCC and 75% for nonbiliary cancers. Although the difference in survival was not statistically significant (P = 0.15), there was a trend toward worse outcomes with biliary cancers.
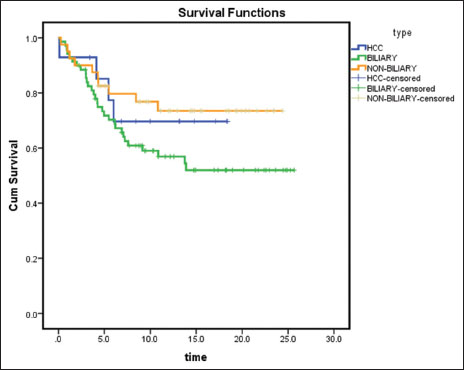
| Figure 10: Kaplan–Meier survival curve of patients at 6 months according to the diagnosis of hepatocellular carcinoma or biliary cancers or nonbiliary cancers (n = 123) (P = 0.15
The pattern of patients' visits to the hospital was analyzed. Of all the patients [Figure 11], 217 (43%) visited the hospital only once, 168 (34%) visited 2–5 times, and 117 (23%) visited more than 5 times.
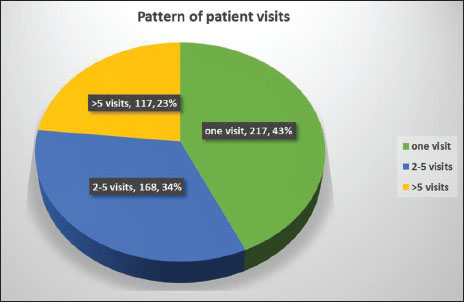
| Figure 11: Pattern of patient visits to hospital
Discussion
This report represents an analysis addressing the epidemiology of cancer patients attending ILBS and a comparison with other Indian and worldwide data.
Hospital-based cancer registry (HBCR) data from the Indian Council of Medical Research (ICMR) showed that overall male-to-female sex ratio % was 116, but a few regions of India such as Dibrugarh (Assam), Bangalore (Karnataka), Chennai (Tamil Nadu), and Thiruvananthapuram (Kerala) registered more female patients. The BRAIRCH (Delhi) registry showed a sex ratio % of 123.[2] Only 12.5% of patients come for treatment in early stages of the disease.[2] In our study, majority of the patients were male and most tumors were advanced at presentation. Most of the patients belonged to Delhi and Uttar Pradesh state due to proximity. Nevertheless, ILBS gets patients from almost the entire country. GBC and HCC were the most common cancers at our institute, reflecting the specialized nature of the hospital. Most of the patients with these cancers were diagnosed in advanced stages. HCC is 2.3 times more common in men than in women, and this difference is consistent globally.[3] In our study, HCC was much more common in men (88.5%), whereas GBC was more common in women (62%).
Besides HBV and HCV, there is a strong association between alcoholic cirrhosis and the development of HCC.[8] HCC has been linked to nonalcoholic fatty liver disease (NAFLD). A US study reported a 2.6% yearly cumulative incidence of HCC in NAFLD and 4% in HCV cirrhosis.[9] A study from Germany identified nonalcoholic steatohepatitis (NASH) as the most common etiology of HCC (24%), surpassing chronic hepatitis C (23.3%), chronic hepatitis B (19.3%), and alcoholic liver disease (12.7%).[10] Diabetes and obesity have been established as independent risk factors for HCC, and that association holds true in the setting of NAFLD and associated NASH.[11] There is also increasing evidence suggesting that NAFLD contributes to noncirrhotic HCC. HCC can develop in patients with metabolic syndrome and NAFLD in the absence of NASH and fibrosis.[12]
The incidence of GBC varies considerably with geographic location.[13] Some of the noted geographic differences may reflect genetic differences. The worldwide occurrence of GBC is < 2/100,000 individuals. Data from Mapuche Indians from Valdivia, Chile, South America show the rate of GBC as 12.3/100,000 for males and 27.3/100,000 for females.[14] American Indians in New Mexico, USA, also have a high average annual rate of GBC (8.9/100,000).[15] The Indo-Gangetic belt, particularly females of northern India (21.5/100,000) and south Karachi, Pakistan (13.8/100,000) has been reported as one of the highest affected regions.[15] GBC is also found in high frequency in Eastern Europe including Poland (14/100,000 in Poland), Czech Republic, and Slovakia and Asia, whereas south Americans of Indian descent (3.7–9.1/100,000), Israel (5/100,000), and Japan (7/100,000) have shown intermediate prevalence of GBC.[16] Mortality from GBC in Chile is 5.2%, the highest in the world.[17]
Population-based data from India had shown that the incidence of GBC was high in Northern Indian cities (5–7/100,000 women) and low (0–0.7/100,000 women) in Southern India. The distribution suggested a high-incidence region comprising the states of Uttar Pradesh, Bihar, Orissa, West Bengal, and Assam.[18] Latest HBCR data from ICMR showed that GBC was in the list of top 10 cancers in men (incidence varied from 2.6%–6% of all cases) in Assam and Jammu and Kashmir, and in women in all the Northern and North-East registries and in Mumbai registry (incidence 4.7%–15.8%). GBC was the most common cancer in women in Silchur registry (Assam). Liver cancer was in the list of top ten cancers in men in registries from Kerala and Assam (incidence 2.7%–11.3%) and in women from Assam (incidence 2.1%).[2]
Population-based registries (PBCR) from ICMR showed that GBC was the ninth-most common cancer in men in Delhi registry (Age-Adjusted Rate [AAR] 5.3) and was among the top ten cancers in registries from Assam, Tripura, and Kolkata (AAR 2.6–8.8). In women GBC was among the top ten cancers in registries from all the Northern and North-East states (AAR 1.8–17.1), including Delhi (AAR 11.8). Liver cancer was in the list of top ten cancers for men in most of the registries (AAR varied from 2 to 38/100,000 population). All the areas covered by Naharlagun PBCR (Arunachal Pradesh) have recorded higher AARs than any other PBCR. Papumpare District (Arunachal Pradesh) had the highest AAR (38.0) within Naharlagun PBCR, as well as overall. For women, liver cancer was the 8th most common cancer in Mumbai registry (AAR 3.4), and among the top ten cancers in Mizoram, Sikkim, and Arunachal Pradesh in the North-East (AAR 5.4–15.4).[2] The dramatic association of GBC with female gender and certain geographical regions (mostly developing countries) has been proposed to be influenced by various female hormones, cholesterol cycling, and salmonella infections.[19],[20]
The incidence of GBC increases with age, with the greatest incidence in persons aged 65 or more, though there are cases of GBC in younger patients worldwide.[21] In our study, GBC was most common in the 35–64 years age group (68.5%), younger than the worldwide prevalence. Established risk factors include gallstone disease, bile composition, calcification of the gallbladder wall, congenital biliary cysts or ductal anatomy, some infections, environmental carcinogens, and drugs. Mortality trends vary. Germany and the Netherlands have relatively high mortality rates from GBC but have shown declines in most age groups. Sweden, France, and Bulgaria show steady upward trends. In Japan, the incidence increased through the 1980s but has stabilized in recent years.[15] GBC incidence in the United States, Britain, and Canada has stabilized or declined. These changes have occurred coincident with the rise in the number of laparoscopic cholecystectomies.[22]
The incidence of GBC is low compared with the incidence of gallstones in the population. Cholecystolithiasis is present in 70%–90% of patients with GBC, and the incidence of GBC in patients with cholecystolithiasis ranges from 0.5% to 3%.[23] In our study, gallstones were present in 44.3% of patients with GBC, 74.2% of whom were women. In addition, gall bladder sludge was present in 7 patients without gallstones with GBC.
At the population level, mortality is inversely related to cholecystectomy rates.[24] A study of northern Indian women reported a benefit of prophylactic cholecystectomy.[25] However, the prevention of GBCs is not considered as a sole indication to perform cholecystectomy in patients who have asymptomatic gallstones. There is a high incidence of GBC in patients with an anomalous pancreaticobiliary duct junction, they may benefit from a prophylactic cholecystectomy.[26] GBC has been associated with partial or complete calcification of the gallbladder wall (porcelain gallbladder),[27] it is associated with cancer in 10%–25% of cases. A calcified or porcelain gallbladder is an indication for cholecystectomy in the asymptomatic patient.
CCAs are cancers of the biliary tract epithelia that are associated with the intrahepatic or extrahepatic bile ducts. Intrahepatic CCAs (iCCAs) are cancers of the biliary tract that arise from intrahepatic bile ducts beyond the second-order ductal system within the liver. The incidence and mortality from iCCAs have been reported to be increasing in the United States and in many other countries.[28],[29]
The incidence of these cancers increases with age, with the majority of patients aged 65 years or older. Patients with defined risk factors such as primary sclerosing cholangitis or choledochal cysts tend to develop tumors at a younger age. These tumors are slightly more common in men. Perihilar CCAs (pCCAs) are cancers that arise from the extrahepatic biliary tract from the second-order ducts to the origin of the cystic duct. These cancers are among the most common malignancies of the biliary tract encountered in many parts of the world. Distal CCAs (dCCAs) are cancers arising from the extrahepatic common hepatic duct between the junction of the cystic duct and the papilla, but not involving either the cystic duct or the ampulla of Vater. dCCAs are far less common than pCCAs and have better treatment outcomes. In our study, 8.8% of patients were diagnosed with CCAs and almost 60% were in an advanced stage.
Pancreatic cancer (ductal adenocarcinoma) is a deadly disease and is currently only curable in a minority of patients with localized and resectable disease. Patients with metastatic disease continue to have a 5-year survival of 2% or less.[30] The most important risk factor for pancreatic cancer is a strong family history. Approximately 10% of all pancreatic cancers are familial, defined as a family history involving at least two affected first-degree relatives (e.g., parents, offspring, and siblings).[31] Smoking tobacco is the best characterized environmental risk factor for pancreatic cancer.[32] The risk decreases for former smokers, and returns to baseline after 20 years of smoking cessation. Occupational risk hazards such as chlorinated hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) correlate with pancreatic cancer and increase relative risk by a comparable degree as smoking.[33] Chlorinated hydrocarbons are associated with dry cleaning and metalwork, and PAHs exposure occurs with metalwork and aluminum production.
Mild or moderate alcohol consumption does not predispose to pancreatic cancer, whereas heavy alcohol consumption (≥9 drinks per day) is associated with an odds ratio (OR) of 1.6.[34] Chronic pancreatitis is a well-known risk factor for pancreatic cancer, with an OR of 2.7 for patients with over 2 years of disease. For patients with < 2 years of chronic pancreatitis, OR is high at 13.6, in large part because pancreatitis in these patients represents a presenting symptom of pancreatic cancer, as opposed to a contributing cause.[35] Type II diabetes mellitus (DM) is associated with a two-fold risk increase for pancreatic cancer. Patients with long-standing DM (> 5 years) have a mildly increased risk compared to patients without DM. As with chronic pancreatitis, the highest risk for pancreatic cancer is in patients with recent onset of DM (< 5 years, and particularly within 1-year), suggesting that DM is principally a manifestation of pancreatic cancer, as opposed to a true risk factor.[36],[37] In our study, 6.8% of patients were diagnosed with pancreatic cancer and majority were in advanced stages.
Pancreatic NETs (pNET) are low-to-intermediate grade neoplasms and have a more indolent course compared to pancreatic adenocarcinoma. The incidence of pNETs in the US was estimated to be 5.6/million in 2010.[38] The incidence of smaller pNETs in the general population is likely to be much higher. In an analysis of 11,472 autopsies performed at a Hong Kong hospital, pNETs were found in 0.1% of all cases.[39] pNETs were slightly more common among men (53%) than women (47%), and the median age at diagnosis was 60 years.[40] At diagnosis, 14% had localized disease, 22% had regional disease, and 64% had distant disease. The survival of patients with pNETs has improved over time.[40] In our study, 2.2% patients had NET and 18.2% of these had pancreas as the primary site.
NETs of the GIT (GI carcinoids) are uncommon. However, they are more common than other primary sites, followed by NETs of the lungs, thymus, and other less common sites such as ovaries, testes, and hepatobiliary system.[40] The incidence of GI NETs is 6.2/100,000 population and has been steadily increasing. Small bowel NETs (midgut) are much more common than both foregut and hindgut.[41] The incidence is approximately 2/100,000/year. Midgut NETs can remain small (< 1 cm) and still metastasize to regional lymph nodes and liver. As these tumors are indolent and patients survive a long time, the prevalence is quite high, making them the second most prevalent GIT tumor, second only to colon cancer. Patients with GI NETs have a higher risk of other noncarcinoid primary tumors.[42] The overall 5-year survival rate of all patients with GI NETs is 28.5%. In our study, 54.5% of the patients with NET had GIT as the primary site. Most of the patients with NET were in stage IV.
Periampullary cancers can arise from biliary as well as pancreatic, duodenal, or ampullary tissues. In our study, 6.2% of the cancers were periampullary. Unlike other cancers, most of them had an early presentation and underwent surgery. It can be difficult to distinguish a primary ampullary carcinoma from other periampullary tumors preoperatively. However, true ampullary cancers have a better prognosis than periampullary malignancies of pancreatic or bile duct origin. Resectability rates are higher, and 5-year survival rates are approximately 30%–50% in patients with limited lymph node involvement. In contrast, < 10% of patients with completely resected node-positive pancreatic cancer are alive at 2 years.
Treatment outcomes and survival for patients with hepatobiliary cancers have not improved much over the past three decades-a period in which a number of successful new treatments have increased patient survival for many other cancers.[43] The mean survival rate for patients with advanced GBC is 6 months, with a 5-year survival rate of < 5% in Stage IV.[44] Patients with CCA usually present at late stages of disease. Approximately half of untreated patients die within 3–4 months of presentation. Few patients are candidates for potentially curative surgical resection at the time of diagnosis. Further, the outcomes after resection with curative intent are poor, with a 5-year survival of ~30%–40% for intrahepatic CCA and ~50% for distal CCA.[45],[46] Many patients are not well enough to undergo aggressive chemotherapy or radiation therapy. Moreover, these biliary cancers respond poorly to these therapies. In a study published in 2010, median survival of 11.7 months was noted when systemic therapy with cisplatin and gemcitabine was used to treat patients with unresectable biliary tract cancers.[47] This combination is presently the standard of care for all biliary tract cancers.
In this study, outcome analysis at 6 months showed a significant decrease in survival with increase in the stage of presentation. Moreover, biliary cancers had a trend toward worse survival as compared to HCC and nonbiliary cancers. This analysis was inadequate due to poor follow-up of patients and incomplete outcome data. Only patients with regular visits to the hospital and follow-up were included. A further prospective study is planned to overcome these deficiencies.
Conclusions
In our study, hepatobiliary cancers showed a predisposition for the male sex, with the majority of patients being in the age group 35–64 years. GBC and HCC were the predominant cancers, with the epidemiology being consistent with the available data from the country. Most of the patients presented in advanced stages (except periampullary cancers), and the risk factors were the same as the available data. Overall survival of patients significantly reduced as stage of disease increased. Further, patients presenting with biliary cancers had worse outcomes. This could be due to the lack of public health awareness, delayed recognition and presentation with advanced and incurable disease. Another factor is lack of follow-up as nearly half the patients had just one visit to the hospital. There is an urgent need to detect these cancers at an early stage to improve patient outcomes. Patients also need to follow medical advice and treatment to have better outcomes. We were unable to assess the reasons for delayed presentation, the economic impact and the role of genetics in our study. However, the study provides robust data for hepatobiliary cancers at a tertiary care hospital.
Conflict of Interest
There are no conflicts of interest.
References
- Available from: https://www.cancer.org/research/cancer-facts- statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Last accessed on 2018 Apr 01].
- National Cancer Registry Programme. Hospital Based (2012-2014) and Population Based (2012-2014) Cancer Registry Reports. New Delhi, India: Indian Council of Medical Research; 18, May 2016
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941-53
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74-108
- Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis 2008; 5: A74
- Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40: 225 –235
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11-30
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387: 349-60
- Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010; 51: 1972-8
- Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G. et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011; 128: 2436-43
- Starley BQ, Calcagno CJ, Harrison SA. et al. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010; 51: 1820-32
- Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis?. Arch Pathol Lab Med 2008; 132: 1761-6
- Wistuba II, Gazdar AF. Gallbladder cancer: Lessons from a rare tumour. Nat Rev Cancer 2004; 4: 695-706
- Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol 2014; 6: 99-109
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int J Cancer 2006; 118: 1591-602
- Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II. et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001; 51: 349-64
- Nervi F, Duarte I, Gómez G, Rodríguez G, Del Pino G, Ferrerio O. et al. Frequency of gallbladder cancer in Chile, a high-risk area. Int J Cancer 1988; 41: 657-60
- Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J Gastroenterol 1999; 18: 24-8
- Pilgrim CH, Groeschl RT, Christians KK, Gamblin TC. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB (Oxford) 2013; 15: 839-44
- Iyer P, Barreto SG, Sahoo B, Chandrani P, Ramadwar MR, Shrikhande SV. et al. Non-typhoidal Salmonella DNA traces in gallbladder cancer. Infect Agent Cancer 2016; 11: 12
- De Aretxabala X, Roa I, Araya JC, Burgos L, Flores P, Wistuba I. et al. Gallbladder cancer in patients less than 40 years old. Br J Surg 1994; 81: 111
- Saika K, Matsuda T. Comparison of time trends in gallbladder cancer mortality (1990-2006) between countries based using the WHO mortality database. Jpn J Clin Oncol 2010; 40: 374-5
- Sinha S, Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978; 147: 929-42
- Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet 1981; 2: 187-9
- Mohandas KM, Patil PS. Cholecystectomy for asymptomatic gallstones can reduce gall bladder cancer mortality in Northern Indian women. Indian J Gastroenterol 2006; 25: 147-51
- Kamisawa T, Tu Y, Kuwata G, Egawa N, Nakajima H, Tsuruta K. Biliary carcinoma risk in patients with pancreaticobiliary maljunction and the degree of extrahepatic bile duct dilatation. Hepatogastroenterology 2006; 53: 816-8
- Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: A relationship revisited. Surgery 2001; 129: 699-703
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001; 33: 1353-7
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002; 2: 10
- Riall TS, Nealon WH, Goodwin JS, Zhang D, Kuo YF, Townsend CMJr. et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg 2006; 10: 1212-23
- Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J 2012; 18: 485-91
- Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks Arch Surg 2008; 393: 535-45
- Andreotti G, Silverman DT. Occupational risk factors and pancreatic cancer: A review of recent findings. Mol Carcinog 2012; 51: 98-108
- Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT. et al. Alcohol consumption and pancreatic cancer: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012; 23: 374-82
- Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA. et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012; 23: 2964-70
- Huxley R, Ansary-Moghaddam A, Berrington de GonzálezA, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer 2005; 92: 2076-83
- Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W. et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011; 47: 1928-37
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063-72
- Lam KY, Lo CY. Pancreatic endocrine tumour: A 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol 1997; 23: 36-42
- Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A. et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007; 14: 3492-500
- de HerderWW, Kwekkeboom DJ, Feelders RA, van AkenMO, Lamberts SW, van der LelyAJ. et al. Somatostatin receptor imaging for neuroendocrine tumors. Pituitary 2006; 9: 243-8
- Mocellin S, Nitti D. Gastrointestinal carcinoid: Epidemiological and survival evidence from a large population-based study (n=25 531). Ann Oncol 2013; 24: 3040-4
- Patel T, Sarin YK. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol 2011; 8: 189-200
- Shukla HS, Sirohi B, Behari A, Sharma A, Majumdar J, Ganguly M. et al. Indian Council of Medical Research. consensus document for the management of gall bladder cancer. Indian J Med Paediatr Oncol 2015; 36: 79-84
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD. et al. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007; 245: 755-62
- Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005; 241: 693-9
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273-81
Address for correspondence
Publication History
Received: 07 September 2018
Accepted: 10 November 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Distribution of all cancers by sex

| Figure 2: Distribution of all cancers by age

| Figure 3: Distribution of all cancers by diagnosis

| Figure 4: Distribution of all cancers by stage at presentation (American Joint Committee on Cancer 2010 TNM staging)

| Figure 5: Distribution of hepatocellular carcinoma by stage at presentation (Barcelona Clinic Liver Cancer staging)

| Figure 6: Distribution of carcinoma gall bladder by stage at presentation (American Joint Committee on Cancer 2010 staging)

| Figure 7: Distribution of carcinoma gall bladder by the presence or absence of gallstones and sex

| Figure 8: Distribution of cholangiocarcinoma, periampullary carcinoma, and carcinoma pancreas by stage of presentation (American Joint Committee on Cancer 2010 staging

| Figure 9: Kaplan–Meier survival curve of patients at 6 months according to the American Joint Committee on Cancer Stage (n = 110) (P < 0.001)

| Figure 10: Kaplan–Meier survival curve of patients at 6 months according to the diagnosis of hepatocellular carcinoma or biliary cancers or nonbiliary cancers (n = 123) (P = 0.15

| Figure 11: Pattern of patient visits to hospital
References
- Available from: https://www.cancer.org/research/cancer-facts- statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Last accessed on 2018 Apr 01].
- National Cancer Registry Programme. Hospital Based (2012-2014) and Population Based (2012-2014) Cancer Registry Reports. New Delhi, India: Indian Council of Medical Research; 18, May 2016
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941-53
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74-108
- Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis 2008; 5: A74
- Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40: 225 –235
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11-30
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387: 349-60
- Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010; 51: 1972-8
- Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G. et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011; 128: 2436-43
- Starley BQ, Calcagno CJ, Harrison SA. et al. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010; 51: 1820-32
- Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis?. Arch Pathol Lab Med 2008; 132: 1761-6
- Wistuba II, Gazdar AF. Gallbladder cancer: Lessons from a rare tumour. Nat Rev Cancer 2004; 4: 695-706
- Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol 2014; 6: 99-109
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int J Cancer 2006; 118: 1591-602
- Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II. et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001; 51: 349-64
- Nervi F, Duarte I, Gómez G, Rodríguez G, Del Pino G, Ferrerio O. et al. Frequency of gallbladder cancer in Chile, a high-risk area. Int J Cancer 1988; 41: 657-60
- Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J Gastroenterol 1999; 18: 24-8
- Pilgrim CH, Groeschl RT, Christians KK, Gamblin TC. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB (Oxford) 2013; 15: 839-44
- Iyer P, Barreto SG, Sahoo B, Chandrani P, Ramadwar MR, Shrikhande SV. et al. Non-typhoidal Salmonella DNA traces in gallbladder cancer. Infect Agent Cancer 2016; 11: 12
- De Aretxabala X, Roa I, Araya JC, Burgos L, Flores P, Wistuba I. et al. Gallbladder cancer in patients less than 40 years old. Br J Surg 1994; 81: 111
- Saika K, Matsuda T. Comparison of time trends in gallbladder cancer mortality (1990-2006) between countries based using the WHO mortality database. Jpn J Clin Oncol 2010; 40: 374-5
- Sinha S, Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978; 147: 929-42
- Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet 1981; 2: 187-9
- Mohandas KM, Patil PS. Cholecystectomy for asymptomatic gallstones can reduce gall bladder cancer mortality in Northern Indian women. Indian J Gastroenterol 2006; 25: 147-51
- Kamisawa T, Tu Y, Kuwata G, Egawa N, Nakajima H, Tsuruta K. Biliary carcinoma risk in patients with pancreaticobiliary maljunction and the degree of extrahepatic bile duct dilatation. Hepatogastroenterology 2006; 53: 816-8
- Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: A relationship revisited. Surgery 2001; 129: 699-703
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001; 33: 1353-7
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002; 2: 10
- Riall TS, Nealon WH, Goodwin JS, Zhang D, Kuo YF, Townsend CMJr. et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg 2006; 10: 1212-23
- Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J 2012; 18: 485-91
- Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks Arch Surg 2008; 393: 535-45
- Andreotti G, Silverman DT. Occupational risk factors and pancreatic cancer: A review of recent findings. Mol Carcinog 2012; 51: 98-108
- Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT. et al. Alcohol consumption and pancreatic cancer: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012; 23: 374-82
- Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA. et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012; 23: 2964-70
- Huxley R, Ansary-Moghaddam A, Berrington de GonzálezA, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer 2005; 92: 2076-83
- Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W. et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011; 47: 1928-37
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063-72
- Lam KY, Lo CY. Pancreatic endocrine tumour: A 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol 1997; 23: 36-42
- Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A. et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007; 14: 3492-500
- de HerderWW, Kwekkeboom DJ, Feelders RA, van AkenMO, Lamberts SW, van der LelyAJ. et al. Somatostatin receptor imaging for neuroendocrine tumors. Pituitary 2006; 9: 243-8
- Mocellin S, Nitti D. Gastrointestinal carcinoid: Epidemiological and survival evidence from a large population-based study (n=25 531). Ann Oncol 2013; 24: 3040-4
- Patel T, Sarin YK. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol 2011; 8: 189-200
- Shukla HS, Sirohi B, Behari A, Sharma A, Majumdar J, Ganguly M. et al. Indian Council of Medical Research. consensus document for the management of gall bladder cancer. Indian J Med Paediatr Oncol 2015; 36: 79-84
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD. et al. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007; 245: 755-62
- Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005; 241: 693-9
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273-81


 PDF
PDF  Views
Views  Share
Share

