Epidemiological and clinical profile of triple negative breast cancer at a cancer hospital in North India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(02): 89-95
DOI: DOI: 10.4103/0971-5851.116185
Abstract
Background: Triple negative breast cancer (TNBC) is a recent concept and the burning topic of research today. Various studies have been reported in western literature on TNBCs or the similar group of basal like cancers, all highlighting the poor prognostic features of this molecular subtype in comparison to the other types of breast cancers. However extensive data from India is lacking. The aim of this study was to analyze the epidemiological and clinical profile of TNBcs at our institute. Materials and Methods: Data on 171 patients of TNBCs registered at this hospital between 2005 and 2008 and followed up until December 2010 was collected and reviewed for epidemiological and clinical features. Results: The median age at presentation was 49 years (22-75 years). Sixty eight patients (40%) had lump in the breast of less than 1 month duration. Fourteen (8%) were nulliparous and 10 (7%) patients had crossed the age of 30 years at first full-term pregnancy, 89 (52%) were pre or peri-menopausal at presentation. Only 8 (5%) patients had a family history of breast or ovarian cancer. One hundred and six (62%) patients were stage II, 26 (15%) stage III, 21 (12%) stage I and 18 (10%) stage IV at presentation. One hundred and twenty eight patients (75%) had early breast cancer eligible for surgery at presentation, 25 (15%) were locally advanced and received neoadjuvant chemotherapy (NACT) and 18 (10%) were found to be metastatic. Modified radical mastectomy was the preferred surgical option by most patients (76%) who underwent upfront surgery in our study. The pathological overall response rates (complete and partial response) after NACT was 75% with complete response rate of 25% and there were no relapses in the complete responders. The median follow-up was 30 months (9-70 months). One hundred and twenty two patients (71%) were alive at last follow-up, 34 (22%) had relapsed, 18 (11%) had died due to progressive disease. Thirty one patients (18%) were lost to follow-up. Most of the relapses were systemic and rarely preceded by local relapses. Conclusions: TNBCs are aggressive cancers with high rates of systemic relapses within the first 3 years of presentation. Longer follow-up of these patients is required for more mature data on these cancers.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Triple negative breast cancer (TNBC) is a recent concept and the burning topic of research today. Various studies have been reported in western literature on TNBCs or the similar group of basal like cancers, all highlighting the poor prognostic features of this molecular subtype in comparison to the other types of breast cancers. However extensive data from India is lacking. The aim of this study was to analyze the epidemiological and clinical profile of TNBcs at our institute.
Materials and Methods:
Data on 171 patients of TNBCs registered at this hospital between 2005 and 2008 and followed up until December 2010 was collected and reviewed for epidemiological and clinical features.
Results:
The median age at presentation was 49 years (22-75 years). Sixty eight patients (40%) had lump in the breast of less than 1 month duration. Fourteen (8%) were nulliparous and 10 (7%) patients had crossed the age of 30 years at first full-term pregnancy, 89 (52%) were pre or peri-menopausal at presentation. Only 8 (5%) patients had a family history of breast or ovarian cancer. One hundred and six (62%) patients were stage II, 26 (15%) stage III, 21 (12%) stage I and 18 (10%) stage IV at presentation. One hundred and twenty eight patients (75%) had early breast cancer eligible for surgery at presentation, 25 (15%) were locally advanced and received neoadjuvant chemotherapy (NACT) and 18 (10%) were found to be metastatic. Modified radical mastectomy was the preferred surgical option by most patients (76%) who underwent upfront surgery in our study. The pathological overall response rates (complete and partial response) after NACT was 75% with complete response rate of 25% and there were no relapses in the complete responders. The median follow-up was 30 months (9-70 months). One hundred and twenty two patients (71%) were alive at last follow-up, 34 (22%) had relapsed, 18 (11%) had died due to progressive disease. Thirty one patients (18%) were lost to follow-up. Most of the relapses were systemic and rarely preceded by local relapses.
Conclusions:
TNBCs are aggressive cancers with high rates of systemic relapses within the first 3 years of presentation. Longer follow-up of these patients is required for more mature data on these cancers.
INTRODUCTION
Breast cancer is the most common cancer among females in urban India and is rapidly catching up with cervical cancer in rural India.[1] It has been long recognized that breast cancer is a heterogeneous disease and not a single entity. Gene expression studies using DNA micro arrays have identified subtypes of breast cancer[2] that were not apparent using traditional histopathologic methods. Four common subtypes have been identified; two of these are derived from estrogen receptor (ER) negative tumors [basal-like and Human epidermal growth factor receptor-2(HER-2) positive] and two are derived from ER-positive tumors (luminal A and B). A fifth subtype called the “Normal” has also been described of which the clinical relevance is not clear.
The definition of basal-like breast cancers has been evolving and though there are no universally agreed upon criteria to define it, the panel developed by Nielsen et al.[3] is generally accepted in practice – basal-like cancers are negative for hormone receptors (HRs) and HER-2, in addition to being positive for cytokeratin (CK) 5/6 or Epidermal growth factor receptor (EGFR). Basal like breast cancers have been found to be more common in younger women of African-American descent, are more aggressive cancers with shorter relapse free survival (RFS), a tendency to visceral rather than bone metastases and a significant likelihood of Breast Cancer (BRCA) susceptibility type-1 mutation.[4] To date, studies on patients with basal-like breast cancers have been limited by small sample sizes and short follow-up times and have been restricted to western literature. To some extent, this is because the basal-like phenotype is based on immunohistochemical staining of tumor slides using anti-keratin antibodies, and these are not yet in general clinical use. In the clinical setting, it has been found that this “basal-like” category of tumors is composed almost entirely of “triple negative breast cancers” (TNBCs)[4] (tumors that are negative for ER, progesterone receptor (PR) and HER2), which can be easily identified in the lab by immunohistochemistry (IHC)[5] and hence has been used as a surrogate for basal-like cancers in many studies all over the world. TNBCs constitute 12-24% of breast cancers[4,6,7] and have attracted the attention of pathologists and oncologists as an easily recognizable prognostic group of breast cancer with aggressive behavior that commonly lack the benefit of any specific targeted therapy. Reliable data on TNBC in Indian setting is scarce[8] and hence, we felt the need to study the clinical profile of these cancers in our setting.
MATERIALS AND METHODS
A total of 171 patients of TNBCs who were registered and treated at a tertiary care cancer hospital in North India between January 2005 and December 2008 were included in this study. All these patients had undergone their primary treatment from this institute and from the very beginning were under the care of a single unit. These patients were followed-up till December 2010. A detailed retrospective analysis was carried out according to a planned Performa. Diagnosis of breast cancer was primarily based on clinical presentation, imaging (mammogram, ultrasound or MRI of the breast when indicated) and cytopathological studies. Staging was done with X-ray chest, Ultrasound abdomen for localized disease with the addition of bone scan and computed tomography (CT) or positron emission tomography (PET) scan for locally advanced disease and metastatic disease. Patients were staged in accordance with American Joint Committee on Cancer (AJCC)-6-(TNM) staging system. TNBC was defined as ER negative, PR negative and HER2 neu negative cancers. These tests were carried out with standard Food and Drug Administration (FDA) approved kits by IHC. For each patient in the database, antibody staining of a set of paraffin embedded slides for ER and PR was carried out. This test was carried out using the BioGenex kit. Any positivity was taken as positive. The over expression of HER2 status was evaluated using the Hercep Test kit from Dako, Denmark. A HER2 report of 3 + by IHC was considered as positive. Confirmation by Flourescence in-situ hybridisation (FISH) was carried out for all those with receptor status 2+. HER2 score of 0 or 1 was considered negative. Patients with ambiguous marker status were excluded. Baseline epidemiological and tumor characteristics of triple negative cancers were analyzed for all 171 patients. Outcomes were analyzed for subgroups of early breast cancer (EBC), locally advanced breast cancer (LABC) and metastatic breast cancer (MBC) separately. EBC was defined as a T-stage ≤ T2 and/or N-stage ≤ N1, LABC was defined as T-stage ≥ T3 and/or N stage ≥ N2 without any evidence of distant metastasis. MBC was defined as any breast cancer with evidence of distant metastasis. However, for survival analysis 31 patients who were lost to follow-up for more than 1 year were excluded. Of these 31 patients, 27 were non metastatic at presentation and were disease free and 4 were metastatic and in remission at last follow-up. Attempts were made to contact these patients telephonically as well as through letters. However, no responses could be obtained. SPSS v. 16 (SPSS Inc.,) was used for statistical analysis. The consort diagram is illustrated in Figure 1.
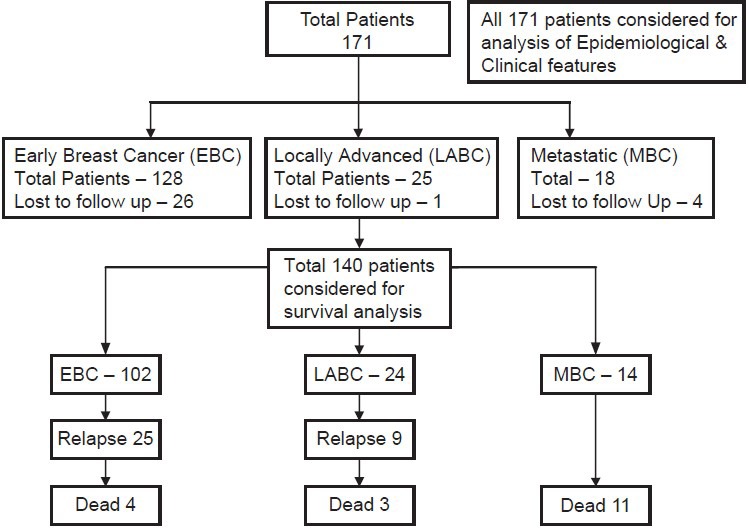
| Fig. 1 Consort diagram of the cohort
RESULTS
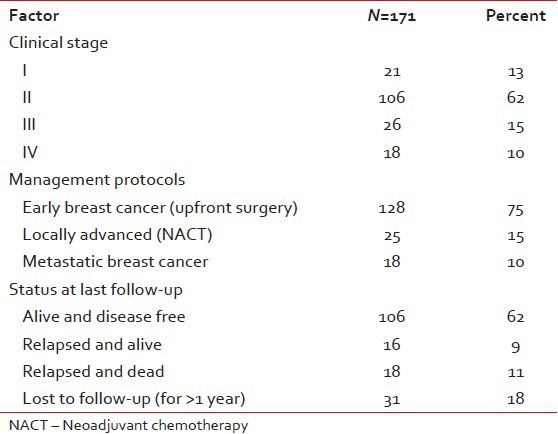
For the period between January 2005 and December 2008, approximately 12.5% of female breast cancers were found to be TNBC's on immunohistochemical analysis of receptor status at our institute. Of these 171 patients satisfying the inclusion criteria were found and included in the study.
Minimum age at presentation was 22 years and maximum age was 75 years with a median age of 49 years. One hundred and three patients (60%) were less than 50 years and only 2 (1.2%) were more than 70 years. Sixty eight patients (40%) had a lump in the breast for less than 1 month duration and 4 (2.5%) did not notice the lump prior to presentation. Other local symptoms like pain (4.3%), redness and ulceration (8.1%) and bloody nipple discharge (1.9%) were seen in only a minor proportion of patients. None of the usual risk factors were found to be significantly elevated in this cohort [Table 1].
Table 1
Risk factors
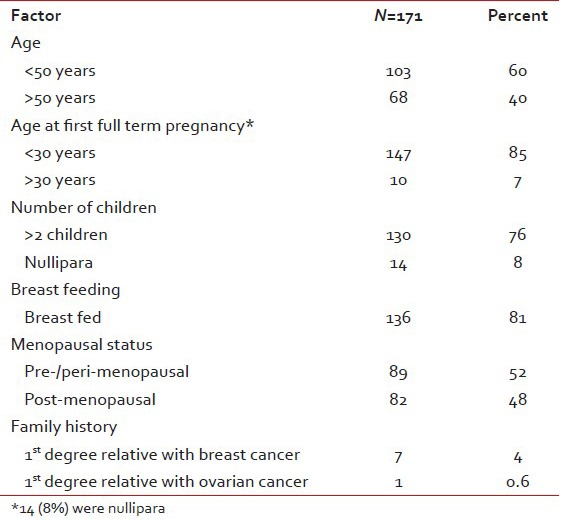
Clinical features
The incidence on the left side and right side was 47.5% and 51.9% respectively and only one patient had bilateral breast cancer at presentation. Clinically T2 was the most common (60.5%) and T4 (10.5%) the least common presentation. Node negative patients were the largest group (65%) followed by N1 (28%), N2 (4%), and N3 (3%). The diagnosis was confirmed by fine needle aspiration cytology (FNAC) prior to definitive surgery in all EBC patients (128/171). The others who were candidates for NACT or had metastases at presentation underwent a trucut biopsy. Two patients developed metachronous second tumor in the opposite breast and 2 second primary cancers (one in the gall bladder and one in ovary). One hundred and twenty two patients (71%) were alive at last follow-up. Eighteen (11%) had died due to progressive disease (PD). Thirty one patients (18%) were lost to follow-up [Table 2].
Table 2
Clinical profile
Early breast cancer
Out of 128 patients who underwent upfront surgery 97 (75.8%) had undergone modified radical mastectomy (MRM) (as per patient wishes) and 31 patients (24.2%) breast conserving surgery (BCS) and the details are summarized in Table 3. Invasive ductal carcinoma was the predominant histology (127 patients) and one patient had metaplastic disease. The mean number of positive lymph nodes was 1.7. Extra capsular extension was present in 26 of 45 patients (58%) who were positive for lymph nodes. Lymphovascular invasion was present in 97 of 128 patients (76%) who underwent surgery. Majority of the tumors (61%) were high grade. The mean size of tumor post-surgery was 3.2 cm (0-9 cm). There was no significant correlation between the size of the tumor and the incidence of lymph node positivity with smaller tumors also showing significant lymph node positivity and larger ones not correspondingly showing any higher incidence of the same [Table 4]. Stage II was the predominant pathological stage (71%) followed by stage III and stage I. Details of adjuvant chemotherapy is summarized in Table 5. The dictum usually followed at our institute was anthracycline based chemotherapy for node negative disease and taxanes with anthracyclines for node positive or large tumors. Univariate analysis showed no overall survival (OS) differences for age (P=0.9), T-size (0.08), lymph node positivity (P=0.5), or pathological stage (P=0.8) most likely due to the low number of events as well as short median follow-up (30 months). However, the RFS was statistically significant for lymph node status (P=0.03) with median RFS of 42 months for lymph node positive group [Figure 2] and for pathological stage [Figure 3] of tumor (P=0.05), whereas it was not significant for age (P=0.3), and size of tumor (P=0.6).
Table 3
Early breast cancer
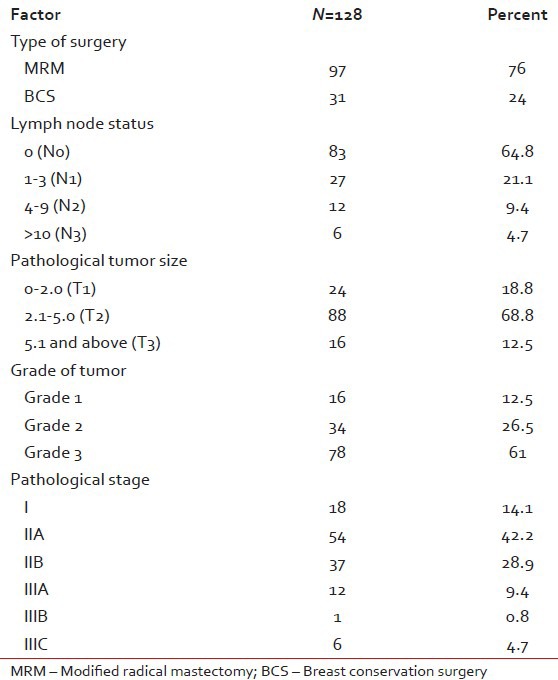
Table 4
Tumor size versus lymph node
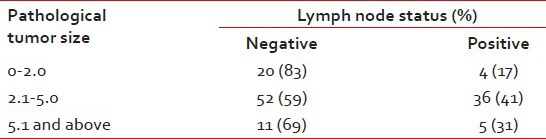
Table 5
Types of adjuvant chemotherapy

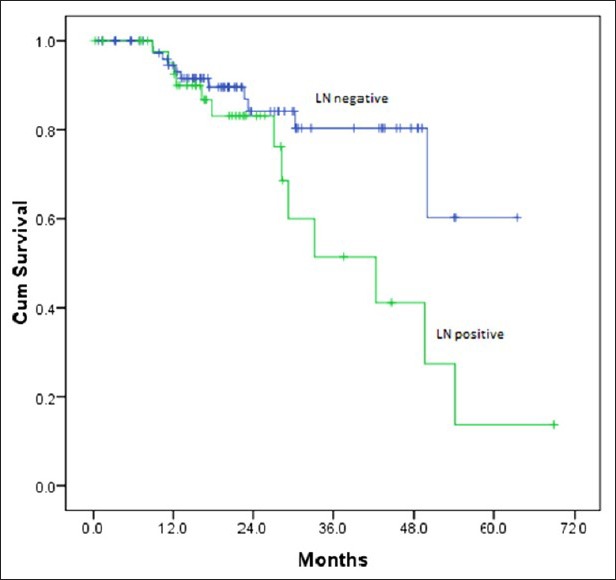
| Fig. 2 Relapse free survival by lymph node status
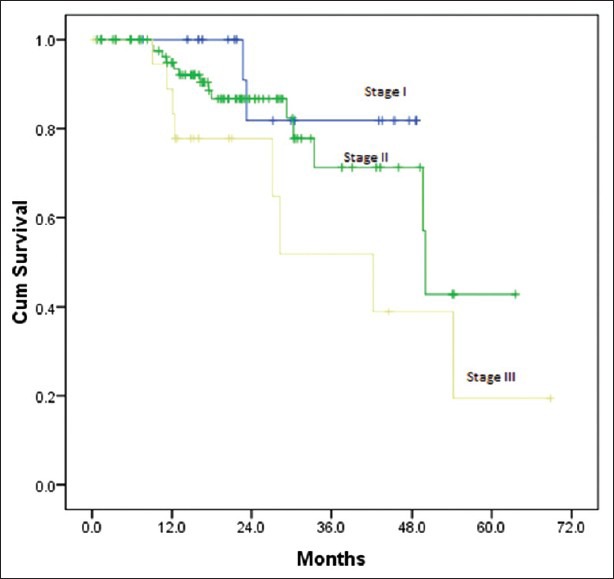
| Fig. 3 Relapse free survival by stage
Locally advanced breast cancer
Twenty five patients (15%) with LABC were given NACT. However, complete data was available for only 24 patients for response evaluation. Majority of the patients were between 40 and 60 years of age (76%), with T4 disease (56%) and variable nodal status. Taxanes with anthracyclines was the preferred neoadjuvant regimen. Fourteen patients (58%) received 3 cycles of NACT, five (21%) patients 4 cycles and four patients (17%) 6 cycles of chemotherapy. Six patients (25%) achieved pathological complete response (pCR) after NACT and 12 (50%) partial response (PR) resulting in overall response rates (ORR) of 75%. the results did not significantly favor any specific type of chemotherapy (P=0.3) [Table 6]. Irrespective of the responses all of them underwent only MRM (strong patient bias towards MRM), followed by adjuvant chemotherapy and radiotherapy. There were no deaths or relapses in patients who were in pathological CR after NACT at a median follow-up of 25 months, which was statistically significant (P=0.04). However, there were 9 relapses and 3 deaths in the non-responders, which was a statistically significant difference (P=0.04 for RFS) [Table 7]. The OS and RFS curves for the two groups are shown in Figures Figures44 and and55.
Table 6
Response to NACT
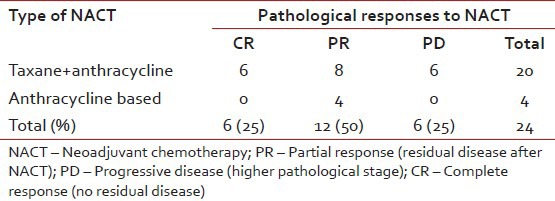
Table 7
Correlation: Response to NACT versus relapse

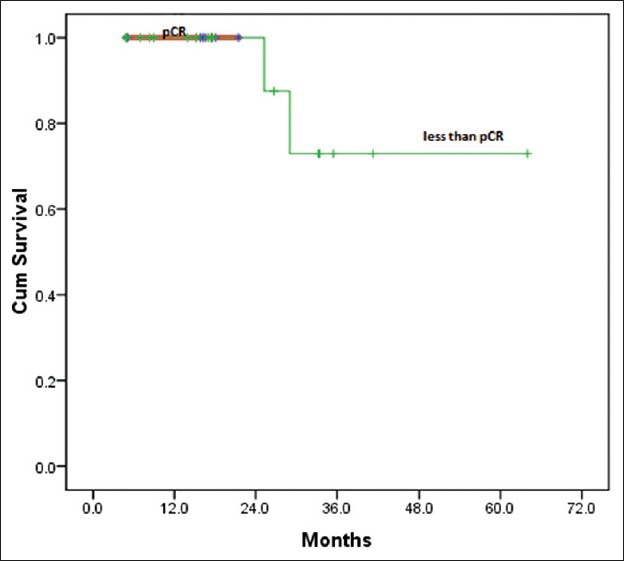
| Fig. 4 Overall survival after neoadjuvant chemotherapy
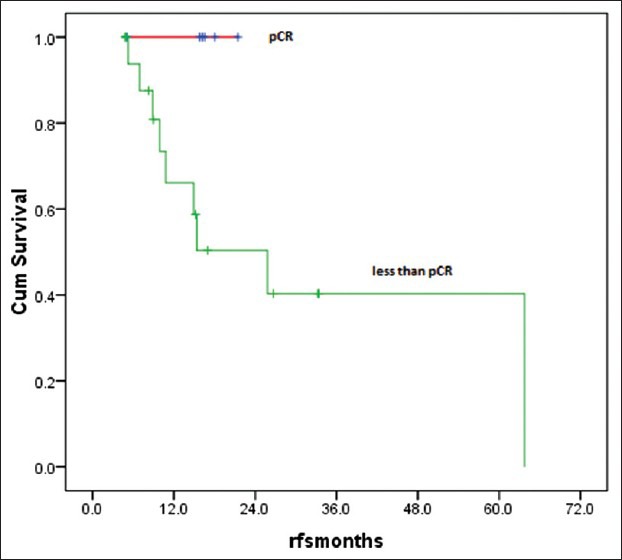
| Fig. 5 Relapse free survival after neoadjuvant chemotherapy
Features of relapse
Thirty four patients (25 in the upfront surgery arm and 9 in the NACT group), out of a total of 153 who were non-metastatic at presentation and underwent surgery relapsed. The different sites of relapse are summarized in Table 8. There was no statistical difference (Chi-square test P=0.9) between the two groups of BCS and MRM patients in terms of the relative frequency or type of relapse [Table 9].
Table 8
Sites of relapse

Table 9
Type of surgery versus relapse

Metastatic breast cancer
There were 18 patients (10.5%) who had metastases at presentation and the sites of metastases are shown in Table 10. It was seen that these patients did not predominantly have large size tumors or significant axillary nodal involvement prior to metastases. Three patients took only brain radiotherapy (RT) and no further treatment. The remaining 15 patients were treated with anthracycline and taxane-based palliative chemotherapy and had an overall response rate (CR + PR) of 75%.
Table 10
Sites of metastases at presentation

Survival analysis for the whole group
The median follow-up time was 30 months (9-70 months). There were 18 deaths with all deaths occurring by the 29th month of follow-up. All the deaths were due to progressive disease and hence the survival curve could be more appropriately termed as breast cancer specific survival (BCSS). Thirty one patients were lost to follow-up and many had entered the study at a later date thus decreasing the median follow-up. The 3 year overall survival was 80% and it plateau thereafter [Figure 6]. Hence the median OS was not reached. On comparison of survival [Figure 7] between Early breast cancer, locally advanced and metastatic disease there was a statistically significant difference (P=0.001). There were 3 (12%) deaths in locally advanced arm, 4 (3.1%) in EBC arm and 11 (61%) in the metastatic arm. The median was reached only for the metastatic arm (15 months). There were 34 relapses totally in the non-metastatic group, 9 (36%) in the locally advanced group and 25 (19.5%) in the EBC group, likely reflecting the higher stage of disease in the locally advanced group. About 50% of the relapses had occurred by the 19th month of follow-up, the median RFS was 29 months (4-48 months) in the locally advanced group and 50 months (44-55 months) in the EBC group [Figure 8] and the difference was statistically significant (P=0.0001).
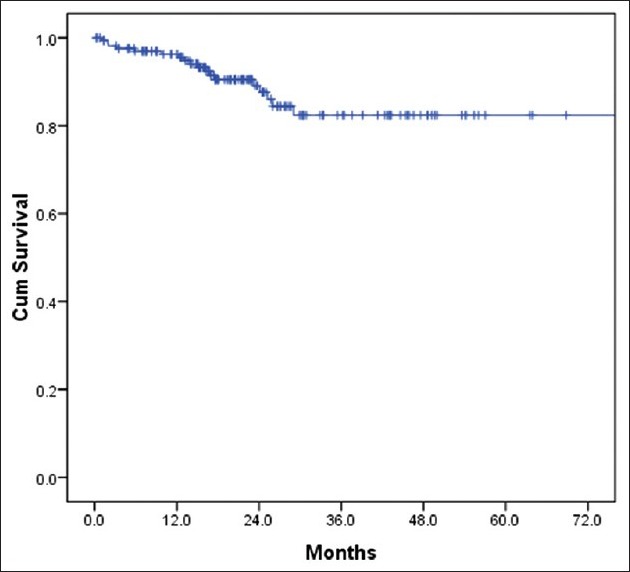
| Fig. 6 Overall survival for whole group
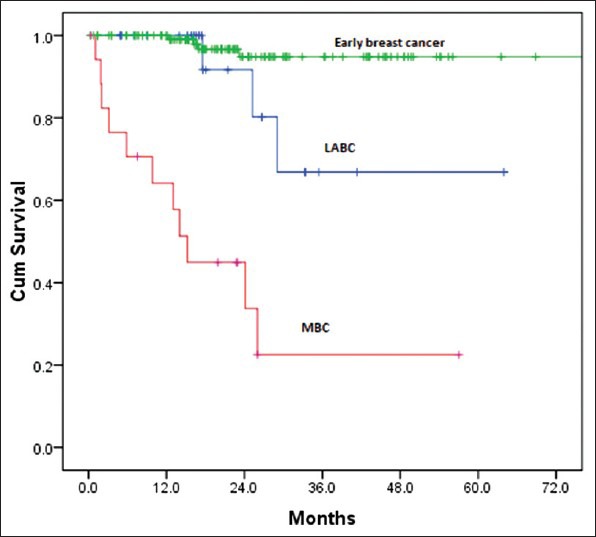
| Fig. 7 Overall survival by groups
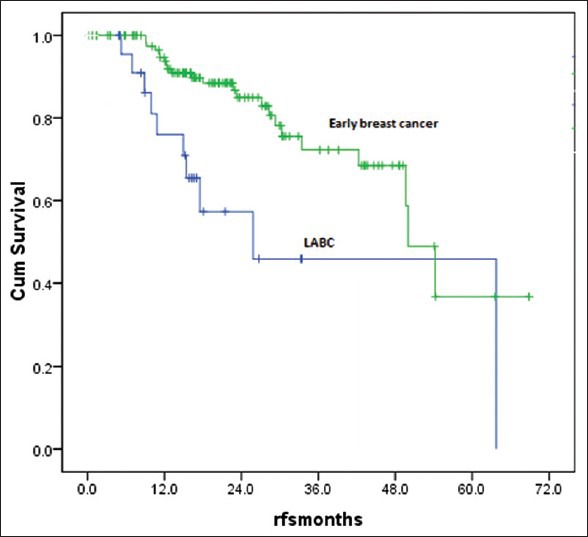
| Fig. 8 Relapse free survival for early and locally advanced disease
DISCUSSION
Our study was designed to look at the demographic profile, clinical features and responses to various modalities of therapies and its correlations to the clinical outcomes in the Indian setting. To our knowledge, this is possibly the largest study on TNBC done in India.
Our population was slightly younger (median age 49 years) than the ones described in western data[4] (median age 53 years). The peak incidence was observed in the 5th decade (36.3%) in our study. This finding of younger median age most likely reflects the general trend of breast cancers occuring a decade earlier in India. None of the standard risk factors for breast cancer had any significant association as was noted in other studies also.[9]
Clinically and pathologically stage II was the most common stage (62%) followed by stage III (15%). The low number of stage IV patients (11%) in our study most probably reflects the bias in presentation to a private tertiary care cancer center. The bias towards MRM was highly significant in our study with all patients of NACT undergoing only MRM. This reflects the social and cultural differences in the Indian population. Irrespective of the type of surgery the relapses were predominantly systemic in our study and very rarely were systemic recurrences preceded by a local relapse. It is well-known that in hormone receptor positive breast cancers, there is a definite increase in the incidence of lymph node positivity with increasing size of the tumor. This has been aptly highlighted in the study by Dent et al.[4] where they have shown that in TNBCs even small tumors have a high chance of lymph node positivity. Our study also found a lack of correlation between tumor size and lymph node positivity, with 41% and 31% node positivity for T2 and T3 size tumors. The T1 size tumors had 17% incidence of lymph node positivity.
TNBCs are known to be highly chemosensitive with higher pCR rates than HR positive tumors. In our study, the pCR rates after NACT of 25% were similar to the results found by Liedtke et al.[10] where they reported 22% CR rates for TNBC. Thirty four out of 153 patients (22%) who underwent surgery (upfront/after NACT) and adjuvant therapy relapsed. Six were local relapses and the rest systemic with only 3 patients with bone only disease and 25 with visceral metastases. Very few local recurrences (16%) preceded a distant recurrence. Most of the recurrences occurred within 2-3 years (84%). The high predilection for visceral metastasis was evident in our study and it was similar to the data from western studies.[4]
Survival
Dent et al.[4] in their study comparing outcomes in triple negative versus other types of breast cancers have clearly shown that TNBC patients were more likely to have died than other patients (42.2% vs. 28%). The median time to death was 4.2 years for patients with TNBCs compared with 6 years for patients with other cancers. In our study, the maximum follow-up period that could be achieved was 70 months. However, the median was 30 months. There were 18 deaths (all related to disease progression) and 34 relapses in this period. The 3 year OS/BCSS and RFS in our study was 80% and 67% respectively. This was comparable to the survival curves for the TNBC group in studies by Dent et al.[4] where the BCSS and RFS were 74% and 67% and by Rakha et al.[6] where BCSS and RFS were 83% and 73% for TNBCs at 5 years.
In the univariate analysis the differences in BCSS/OS, though present were not statistically significant for known prognostic factors such as age, pathological tumor size, and nodal status in patients who underwent upfront surgery likely due to low number of events and short median follow-up. However, there was a significant difference in RFS for lymph node positive and negative groups (P=0.03) and pathological stage of disease (P=0.05). The 3 year RFS for lymph node negative and positive patients was 80% and 53%. The 3 year RFS for stage II and III patients was 70% and 50% respectively. Rakha et al.[6] have noted 5 year RFS of 67% for TNBC patients who were lymph node negative.
In the NACT group, there was a statistically significant difference for both BCSS and RFS between the complete responders and non-responders. The 3 year BCSS and RFS for the non-responders was 70% and 40%. The follow-up period was short (maximum of 3 years), and there were no relapses or deaths in the complete responders (P=0.04). Similar results were seen in the study by Liedtke et al.[10] with 94% 3 year OS for the pathological complete responders and 68% for patients with residual disease.
It is likely that a longer follow-up period would have brought out the differences in OS statistics for relevant prognostic factors. However, the numbers of relapses were sufficient to show statistical significance for the prognostic factors such as stage and lymph node positivity.
CONCLUSIONS
Triple negative cancers are highly aggressive tumors that have distinct epidemiological, pathological and outcome characteristics.[4,6,7] This was evident in our study where we found higher grade tumors with a significant number of early relapses despite the short median follow-up. About 12.5% of breast cancers at this institute were TNBCs, and they affected younger females with no significant risk factors or family history, with no correlation between lymph node positivity and tumor size, had most of the recurrences distally in visceral organs within a period of 3 years. They responded well to NACT and the complete responders were relapse free at 3 years of follow-up. These data are still early findings and further follow-up for more mature data is on.
The main limitation of our study was the lack of testing for basal cytokeratins. Further, large scale prospective trials incorporating basal cytokeratin markers and gene expression profiling are required for complete characterization of these tumors and to identify a positive marker that can facilitate targeted therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Consort diagram of the cohort

| Fig. 2 Relapse free survival by lymph node status

| Fig. 3 Relapse free survival by stage

| Fig. 4 Overall survival after neoadjuvant chemotherapy

| Fig. 5 Relapse free survival after neoadjuvant chemotherapy

| Fig. 6 Overall survival for whole group

| Fig. 7 Overall survival by groups

| Fig. 8 Relapse free survival for early and locally advanced disease


 PDF
PDF  Views
Views  Share
Share

