Epstein–Barr Virus Infection in Adult Patients with Gastric Cancer in Northeast of Iran
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 206-209
DOI: DOI: 10.4103/ijmpo.ijmpo_132_17
Abstract
Background: Epstein–Barr virus (EBV) is a DNA virus from human herpes virus that associates with many of the human cancers including gastric cancer (GC). Aims: The aim of the present study was to report infection of EBV in adult patients with GC in Northeast of Iran and the correlation between a number of clinicopathology factors with EBV status. Materials and Methods: In a case–control study in 2016, 56 GC patients and 56 controls were selected for the analysis. All patients had gastric adenocarcinoma untreated patients with age >18 years. EBV status detected by the polymerase chain reaction method. Results: Out of 56 GC patients, 35 (62.5%) were EBV positivity and out of 56 controls 3 (5.4%) were EBV positivity (P < 0 class="i" xss=removed>P > 0.05). Furthermore, the progression-free survival rate for the patients with EBV negativity was 95.2% compared with 82.9% for EBV positivity (P = 0.174). Conclusions: This study reported a very high prevalence of EBV-associated GC in the Northeast of Iran compared with other areas of the World and showed a significant correlation between EBV infection and incidence of GC.
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Epstein–Barr virus (EBV) is a DNA virus from human herpes virus that associates with many of the human cancers including gastric cancer (GC). Aims: The aim of the present study was to report infection of EBV in adult patients with GC in Northeast of Iran and the correlation between a number of clinicopathology factors with EBV status. Materials and Methods: In a case–control study in 2016, 56 GC patients and 56 controls were selected for the analysis. All patients had gastric adenocarcinoma untreated patients with age >18 years. EBV status detected by the polymerase chain reaction method. Results: Out of 56 GC patients, 35 (62.5%) were EBV positivity and out of 56 controls 3 (5.4%) were EBV positivity (P < 0 class="i" xss=removed>P > 0.05). Furthermore, the progression-free survival rate for the patients with EBV negativity was 95.2% compared with 82.9% for EBV positivity (P = 0.174). Conclusions: This study reported a very high prevalence of EBV-associated GC in the Northeast of Iran compared with other areas of the World and showed a significant correlation between EBV infection and incidence of GC.
Introduction
Gastric cancer (GC) is the third most common cause of malignancy death worldwide that has estimated more than 700,000 deaths in 2012.[1] Advanced stage, older age, cardiac tumor localization, and less differentiated histology are adverse prognostic indicators in the patients with GC.[2],[3],[4] Epstein–Barr virus (EBV) is a ubiquitous double-stranded DNA virus from human herpes virus family, which has B-lymphotropism [5] and this virus has been shown to associate with many of the human malignancies including GC.[6] Around 10% of the GCs throughout the world are monoclonal proliferations of EBV-carrying tumor cells [7] that the lowest prevalence is in Papua New Guinea, Pakistan, and Korea, between 1% and 3% and the highest in Germany and the USA, between 16% and 18%.[6] Although the polymerase chain reaction (P CR) had been used in the first detection of EBV in GC, the majority of studies have used RNA in situ hybridization (ISH) due to the possibility of viral genome amplification from infected lymphocytes infiltrating the tumor, resulting in a false positive. Still, there are few studies that use and compare both techniques for EBV detection.[8] The aim of the present study was to report infection of EBV in adult patients with GC in Northeast of Iran and the correlation between a number of clinicopathology factors with EBV status.
Materials and Methods
Patients
In 2016 and in a case-control study, out of all cancer patients referred to Emam Reza Hospital, Mashhad, Iran, the GC was confirmed in 56 patients after endoscopy and biopsy, and after gastrectomy, these patients were selected as case group. We selected 56 controls that had no GC. The inclusion criteria: all of gastric adenocarcinoma untreated patients with age >18 years. The exclusion criteria: The patients with severe gastritis or atrophic and nonadenocarcinoma cancer and treated patient. The progression-free survival (P FS) is defined as the start time of treatment to disease progression or death from any cause.
Hematoxylin and eosin staining
At first, all of the samples were evaluated by a pathologist and hematoxylin and eosin staining was done on 3–4 micron slicing of the sample to confirm adenocarcinoma that after that, adenocarcinoma sampling selected as the proper paraffin blocks or sections for PCR detection.
Polymerase chain reaction detection
Paraffin blocks were sectioned by microtome 2 μm about 20 times. Then, these materials gathered in a microtube and are done deparaffinization and rehydaration. After that, DNA extraction by columnar device (Takapozist, Iran) was done and DNA prepared for real-time PCR. EBV DNA detection was fulfilled through real-time PCR system (Bioneer, South Korea), with the real-time PCR kit (Takapozist, Iran) and according to positive and negative control, the data were acquired.
Statistical analysis
The data analyzed with SPSS version 22 software (IBM Corp., Armonk, NY, USA). T-test was used for comparison of means between two groups and Chi-square test for other variables. The results of PCR were also checked by univariate and multivariate logistic regression. P < 0>
Results
Out of 56 GC patients with mean age of 66.2 years (±9.8), 45 patients (80.4%) were males and 35 (62.5%) were EBV positivity based on PCR. In addition, out of 56 controls with the mean age of 57.9 years (±14.4), 21 (37.5%) were males and 3 (5.4%) were EBV positivity. There was a significant difference between two groups for EBV status (P < 0 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_132_17#TB_1" xss=removed>Table 1] shows the clinicopathological characteristics of GC patients based on the results of PCR for EBV. There is not a significant correlation between the variables with the EBV status (P > 0.05).
|
Variables |
EBV-positive (n=35), n (%) |
EBV-negative (n=21), n (%) |
χ2 |
P |
|---|---|---|---|---|
|
EBV – Epstein‑Barr virus |
||||
|
Age (years) |
65.9±10.4 |
66.76±8.74 |
0.30 |
0.765 |
|
Sex |
9.52 |
0.999 |
||
|
Male |
28 (80) |
17 (81) |
||
|
Female |
7 (20) |
4 (19) |
||
|
Anatomic location |
0.15 |
0.999 |
||
|
Cardia and fundus |
4 (11.4) |
2 (9.5) |
||
|
Antrum and pylorus |
12 (34.3) |
7 (33.4) |
||
|
Corpus (body) |
19 (54.3) |
12 (57.1) |
||
|
Extension |
0.37 |
0.923 |
||
|
Muscle |
5 (14.3) |
2 (9.5) |
||
|
Serous |
7 (20) |
5 (23.8) |
||
|
Lymph nodes |
23 (65.7) |
14 (66.7) |
||
|
Stage |
3.02 |
0.374 |
||
|
I |
6 (17.1) |
3 (14.3) |
||
|
II |
6 (17.1) |
3 (14.3) |
||
|
III |
23 (65.7) |
13 (61.9) |
||
|
IV |
0 |
2 (9.5) |
||
|
Grade |
0.17 |
0.881 |
||
|
High |
9 (25.7) |
6 (28.6) |
||
|
Moderate |
17 (48.6) |
9 (42.8) |
||
|
Low |
9 (25.7) |
6 (28.6) |
||
|
Variables |
Unadjusted |
Adjusted |
||
|---|---|---|---|---|
|
OR (95% CI) |
P |
OR (95% CI) |
P |
|
|
OR – Odds ratio; CI – Confidence interval |
||||
|
Age (years) |
1.005 (0.948-1.06) |
0.878 |
1.001 (0.938-1.06) |
0.978 |
|
Gender |
||||
|
Male |
Reference |
Reference |
||
|
Female |
0.938 (0.236-3.724) |
0.927 |
1.012 (0.24-4.26) |
0.987 |
|
Extension |
||||
|
Lymph nodes |
Reference |
Reference |
||
|
Muscle |
1.522 (0.259-8.92) |
0.642 |
0.692 (0.009-55.8) |
0.870 |
|
Serous |
0.852 (0.226-3.20) |
0.813 |
0.266 (0.015-4.81) |
0.371 |
|
Grading |
||||
|
Low |
Reference |
Reference |
||
|
High |
1 (0.232-4.31) |
0.999 |
0.762 (0.134-4.32) |
0.759 |
|
Moderate |
1.25 (0.339-4.67) |
0.730 |
1.184 (0.24-5.72) |
0.834 |
|
Stage |
||||
|
I |
Reference |
Reference |
||
|
II |
1 (0.141-7.09) |
0.999 |
2.46 (00.03-154.33) |
0.669 |
|
III |
0.885 (0.18-4.14) |
0.876 |
3.657 (0.154-86.91) |
0.423 |
|
Location |
||||
|
Corpus |
Reference |
Reference |
||
|
Cardia and fundus |
1.083 (0.333-3.522) |
0.895 |
0.96 (0.21-4.29) |
0.960 |
|
Antrum and pyloric |
1.263 (0.2-7.99) |
0.804 |
3.735 (0.26-53.26) |
0.331 |
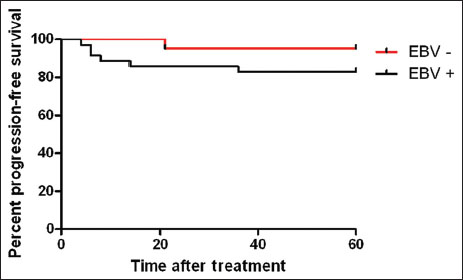
| Figure. 1The 5-year progression-free survival for the patients with gastric cancer
Discussion
This case–control study showed that the prevalence of EBV in GC patients was significantly more than controls in Northeastern Iran (62.5% vs. 5.4%). Furthermore, the analyses did not show a significant correlation between clinicopathological figures of GC with EBV status. A meta-analysis among 39 case–control studies published up to October 2015, reported that EBV infection increases significantly the risk of GC,[9] but other meta-analysis by Lee et al.[10] reported that EBV infection is not associated with the incidence of this cancer in Asians on the analysis of 48 studies published up to December 2007. Murphy et al.[11] showed that the prevalence of EBV-positive GC did not differ according to a geographical area. A systematic review from Chen et al.[12] on published articles up to September 2014, showed that evidence based on ISH method strongly suggests an association between EBV infection and GC risk, but the PCR method alone is invalid to confirm such association. Several studies [7],[13],[14] indicated that EBV-associated GCs include about 10% of all GCs in the World. One study [15] reported that the incidence of EBV-associated GC in all cases of GC is distributed from highest (16%–18%) in the USA and Germany to the lowest (4.3%) in China. Two studies in Iran [6],[16] reported that the prevalence of EBV in GC patients was 6.66% and 3%, respectively, and concluded that the frequency of EBV-associated GC in Iran [6],[16] and the Middle East [6] was low, but this study reported that the prevalence of EBV in GC was 62.5% by PCR method and very higher than other studies in Iran. Camargo et al.[17] among 4599 patients with invasive GC, suggested that tumor EBV positivity is an additional prognostic indicator in GC and 8.2% tumors were EBV-positive overall. Koshiol et al.[18] did not find a positive association between prediagnostic EBV seropositivity and GC and in fact, there was some evidence that EBV seropositivity was associated with a reduced risk of malignancy and death after diagnosis of cardia cancer. The unadjusted logistic regression analyses showed tumor EBV positivity was higher in an early stage, cardiac localization, diffuse-type histology, poorer differentiation and men that there was a direct correlation between stage and mortality.[17] On the contrary, a meta-analysis by Li et al.[19] found a significant risk for lymph node spread. EBV-positive GC also displays distinct clinical, genetic and demographic features as compared to EBV-negative cancer.[20],[21] Differences in prevalence and more generally the EBV infection patterns have never been clearly associated with race, but merely seen as differences in socioeconomic, hygienic, and cultural behavior.[22] Vo et al.[23] checked EBV in GCs and suggested that there are ethnic differences in tumor virology and pathogenesis and two other studies confirmed it.[24],[25] Camargo et al.[26] reported that smoking is associated with risk of EBV positive in GC. Therefore, these differences in EBV-associated GC incidence in different areas may reflect the epidemiologic and clinicopathologic factors; dietary habits and genetic. A meta-analysis revealed that patients with EBV-associated GC had a longer survival than those with EBV-negative GC [17] that two other studies confirmed this result.[27],[28] This study showed that EBV-associated GC patients had a low PFS rate compared with EBV-negative GC patients (P > 0.05). While some studies have shown significantly better prognosis in EBV-associated GC than in EBV-negative GC.[17],[29],[30]
Conclusions
This study reported a very high prevalence of EBV-associated GC in the Northeast of Iran compared with other areas of the World and showed a significant correlation between EBV infection and incidence of GC. The further studies need more cases in other areas of Iran by controlling epidemiologic and clinicopathologic factors; dietary habits and genetic that can receive the effect of each of these factors on the prevalence of EBV-associated GC.
Conflict of Interest
There are no conflicts of interest.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11.Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Last accessed on 2016 June 16]
- Baghestani AR, Hajizadeh E, Fatemi SR. Parametric model to analyse the survival of gastric cancer in the presence of interval censoring. Tumori 2010; 96: 433-7
- Zhu HP, Xia X, Yu CH, Adnan A, Liu SF, Du YK. et al. Application of Weibull model for survival of patients with gastric cancer. BMC Gastroenterol 2011; 11: 1
- Cammerer G, Formentini A, Karletshofer M, Henne-Bruns D, Kornmann M. Evaluation of important prognostic clinical and pathological factors in gastric cancer. Anticancer Res 2012; 32: 1839-42
- Rickinson AB, Kieff E. Epstein-Barr virus In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B. et al Fields Virology 4th ed. Philadelphina: Philadelphina: Lippincott Williams & Wilkins; 2012. 2. 2575-628
- Abdirad A, Ghaderi-Sohi S, Shuyama K, Koriyama C, Nadimi-Barforoosh H, Emami S. et al. Epstein-barr virus associated gastric carcinoma: A report from Iran in the last four decades. Diagn Pathol 2007; 2: 25
- Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol 1992; 140: 769-74
- de LimaMA, Ferreira MV, Barros MA, Pardini MI, Ferrasi AC. et al. Epstein-Barr virus-associated gastric carcinoma in Brazil: Comparison between in situ hybridization and polymerase chain reaction detection. Braz J Microbiol 2012; 43: 393-404
- Bae JM, Kim EH. Epstein-Barr virus and gastric cancer risk: A Meta-analysis with meta-regression of case-control studies. J Prev Med Public Health 2016; 49: 97-107
- Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J Gastroenterol Hepatol 2009; 24: 354-65
- Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009; 137: 824-33
- Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine (Baltimore) 2015; 94: e792
- Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. et al. Epstein-Barr virus in gastric carcinoma. Am J Pathol 1993; 143: 1250-4
- Takada K, Sarin YK. Epstein-Barr virus and gastric carcinoma. Mol Pathol 2000; 53: 255-61
- Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R. et al. Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. Br J Cancer 2011; 105: 38-43
- Faghihloo E, Saremi MR, Mahabadi M, Akbari H, Saberfar E. Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran. Arch Iran Med 2014; 17: 767-70
- Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan KM, Kim AH, Matsuo K. et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: An international pooled analysis. Gut 2014; 63: 236-43
- Koshiol J, Qiao YL, Mark SD, Dawsey SM, Abnet CC, Kamangar F. et al. Epstein-Barr virus serology and gastric cancer incidence and survival. Br J Cancer 2007; 97: 1567-9
- Li S, Du H, Wang Z, Zhou L, Zhao X, Zeng Y. et al. Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci 2010; 53: 524-30
- Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R. et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res 2011; 71: 7187-97
- Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: Epidemiological and clinicopathological features. Cancer Sci 2008; 99: 195-201
- Hjalgrim H, Friborg J, Melbye M. The epidemiology of EBV and its association with malignant disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R. et al. editor Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Ch. 53. Cambridge: Cambridge: Cambridge University Press; 2007
- Vo QN, Geradts J, Gulley ML, Boudreau DA, Bravo JC, Schneider BG. et al. Epstein-Barr virus in gastric adenocarcinomas: Association with ethnicity and CDKN2A promoter methylation. J Clin Pathol 2002; 55: 669-75
- Laurini JA, Perry AM, Boilesen E, Diebold J, Maclennan KA, Müller-Hermelink HK. et al. Classification of non-Hodgkin lymphoma in central and South America: A review of 1028 cases. Blood 2012; 120: 4795-801
- Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol 2014; 99: 227-39
- Camargo MC, Koriyama C, Matsuo K, Kim WH, Herrera-Goepfert R, Liao LM. et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. Int J Cancer 2014; 134: 948-53
- van BeekJ, Zur HausenA, Klein KranenbargE, van de VeldeCJ, Middeldorp JM, van den BruleAJ. et al. EBV-positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol 2004; 22: 664-70
- Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki KangW. et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 2010; 139: 84-9200
- Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer 1994; 73: 2239-49
- Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y. et al. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer 1990; 66: 945-52
Address for correspondence
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure. 1The 5-year progression-free survival for the patients with gastric cancer
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11.Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Last accessed on 2016 June 16]
- Baghestani AR, Hajizadeh E, Fatemi SR. Parametric model to analyse the survival of gastric cancer in the presence of interval censoring. Tumori 2010; 96: 433-7
- Zhu HP, Xia X, Yu CH, Adnan A, Liu SF, Du YK. et al. Application of Weibull model for survival of patients with gastric cancer. BMC Gastroenterol 2011; 11: 1
- Cammerer G, Formentini A, Karletshofer M, Henne-Bruns D, Kornmann M. Evaluation of important prognostic clinical and pathological factors in gastric cancer. Anticancer Res 2012; 32: 1839-42
- Rickinson AB, Kieff E. Epstein-Barr virus In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B. et al Fields Virology 4th ed. Philadelphina: Philadelphina: Lippincott Williams & Wilkins; 2012. 2. 2575-628
- Abdirad A, Ghaderi-Sohi S, Shuyama K, Koriyama C, Nadimi-Barforoosh H, Emami S. et al. Epstein-barr virus associated gastric carcinoma: A report from Iran in the last four decades. Diagn Pathol 2007; 2: 25
- Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol 1992; 140: 769-74
- de LimaMA, Ferreira MV, Barros MA, Pardini MI, Ferrasi AC. et al. Epstein-Barr virus-associated gastric carcinoma in Brazil: Comparison between in situ hybridization and polymerase chain reaction detection. Braz J Microbiol 2012; 43: 393-404
- Bae JM, Kim EH. Epstein-Barr virus and gastric cancer risk: A Meta-analysis with meta-regression of case-control studies. J Prev Med Public Health 2016; 49: 97-107
- Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J Gastroenterol Hepatol 2009; 24: 354-65
- Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009; 137: 824-33
- Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine (Baltimore) 2015; 94: e792
- Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. et al. Epstein-Barr virus in gastric carcinoma. Am J Pathol 1993; 143: 1250-4
- Takada K, Sarin YK. Epstein-Barr virus and gastric carcinoma. Mol Pathol 2000; 53: 255-61
- Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R. et al. Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. Br J Cancer 2011; 105: 38-43
- Faghihloo E, Saremi MR, Mahabadi M, Akbari H, Saberfar E. Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran. Arch Iran Med 2014; 17: 767-70
- Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan KM, Kim AH, Matsuo K. et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: An international pooled analysis. Gut 2014; 63: 236-43
- Koshiol J, Qiao YL, Mark SD, Dawsey SM, Abnet CC, Kamangar F. et al. Epstein-Barr virus serology and gastric cancer incidence and survival. Br J Cancer 2007; 97: 1567-9
- Li S, Du H, Wang Z, Zhou L, Zhao X, Zeng Y. et al. Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci 2010; 53: 524-30
- Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R. et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res 2011; 71: 7187-97
- Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: Epidemiological and clinicopathological features. Cancer Sci 2008; 99: 195-201
- Hjalgrim H, Friborg J, Melbye M. The epidemiology of EBV and its association with malignant disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R. et al. editor Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Ch. 53. Cambridge: Cambridge: Cambridge University Press; 2007
- Vo QN, Geradts J, Gulley ML, Boudreau DA, Bravo JC, Schneider BG. et al. Epstein-Barr virus in gastric adenocarcinomas: Association with ethnicity and CDKN2A promoter methylation. J Clin Pathol 2002; 55: 669-75
- Laurini JA, Perry AM, Boilesen E, Diebold J, Maclennan KA, Müller-Hermelink HK. et al. Classification of non-Hodgkin lymphoma in central and South America: A review of 1028 cases. Blood 2012; 120: 4795-801
- Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol 2014; 99: 227-39
- Camargo MC, Koriyama C, Matsuo K, Kim WH, Herrera-Goepfert R, Liao LM. et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. Int J Cancer 2014; 134: 948-53
- van BeekJ, Zur HausenA, Klein KranenbargE, van de VeldeCJ, Middeldorp JM, van den BruleAJ. et al. EBV-positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol 2004; 22: 664-70
- Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki KangW. et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 2010; 139: 84-9200
- Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer 1994; 73: 2239-49
- Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y. et al. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer 1990; 66: 945-52


 PDF
PDF  Views
Views  Share
Share

