Evaluation of Cytotoxicity Effects of Oleo-Gum-Resin and Its Essential Oil of Ferula assa?foetida and Ferulic Acid on 4T1 Breast Cancer Cells
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 116-120
DOI: DOI: 10.4103/ijmpo.ijmpo_60_16
Abstract
Background: Cancer causes significant morbidity and mortality and is a major public health problem worldwide. Breast cancer is a leading cause of cancer-associated mortality in women, and the incidence is also on the rise in the entire world. Medicinal plants have been an important source of several clinically useful anticancer agents. Aim: In this study, we studied the growth inhibitory effect of asafoetida and its essential oil and ferulic acid on antitumor activity using mouse breast cancer cell line. Materials and Methods: For this aim, cells were exposed to these components at different concentrations and for different time durations. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out to characterize the cytotoxicity of the constituents used. Results: Our results showed that all three constituents could inhibit 4T1 cell proliferation. Our MTT assay results showed a significant cytotoxicity effect in a time- and concentration-dependent manner. It also demonstrated that essential oil of asafoetida has a stronger effect in decreasing viability breast cancer cells. Ferulic acid showed a significant effect only at a dose of 500 μg/ml. Conclusions: Based on the results of cellular carried out in this study, we could demonstrate that asafoetida and its essential oil and ferulic acid have inhibitory effect on the growth of breast cancer cell line. As evidenced from these preliminary results, asafoetida and its derivative constituents may be considered as attractive alternatives to serve as lead compounds in drug development for breast cancer as an adjuvant therapy. However, much remains to be done before such agent could be introduced to the clinic.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Cancer causes significant morbidity and mortality and is a major public health problem worldwide. Breast cancer is a leading cause of cancer-associated mortality in women, and the incidence is also on the rise in the entire world. Medicinal plants have been an important source of several clinically useful anticancer agents.
Aim:
In this study, we studied the growth inhibitory effect of asafoetida and its essential oil and ferulic acid on antitumor activity using mouse breast cancer cell line.
Materials and Methods:
For this aim, cells were exposed to these components at different concentrations and for different time durations. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out to characterize the cytotoxicity of the constituents used.
Results:
Our results showed that all three constituents could inhibit 4T1 cell proliferation. Our MTT assay results showed a significant cytotoxicity effect in a time- and concentration-dependent manner. It also demonstrated that essential oil of asafoetida has a stronger effect in decreasing viability breast cancer cells. Ferulic acid showed a significant effect only at a dose of 500 μg/ml.
Conclusions:
Based on the results of cellular carried out in this study, we could demonstrate that asafoetida and its essential oil and ferulic acid have inhibitory effect on the growth of breast cancer cell line. As evidenced from these preliminary results, asafoetida and its derivative constituents may be considered as attractive alternatives to serve as lead compounds in drug development for breast cancer as an adjuvant therapy. However, much remains to be done before such agent could be introduced to the clinic.
Introduction
Cancer is a major public health problem in many parts of the world. It is currently the second leading cause of death and is expected to surpass heart diseases as the leading cause of death in the next few years.[1] Breast cancer is the most common type of cancer among women, especially in industrialized countries.[2] Despite the advance of cancer prevention including better diagnostic for early detection and used of antiestrogenic drugs such as tamoxifen and raloxifene, which resulted in the reduction of breast cancer incident and mortality, breast cancer is still the most commonly diagnosed cancer that is associated with mortality in women.[3] The conventional therapies for cancer include chemo- and radiotherapies mediated by inducing apoptosis or inhibiting proliferation in neoplastic cells.[4] These therapies cause damage to healthy tissues around the tumors[5] and also develop resistance by numerous tumors.[6] In the recent years, researchers have been studying alternatives of cancer therapy by applying potential biological molecules to target neoplastic tumors.[7] Herbs have been identified as an important source of novel bioactive compounds for medicine development including cancer chemotherapeutic drugs.[8] Ferula assa-foetida L. which belongs to Apiaceae family is an herbaceous perennial with an unpleasant odor that grows wildly in central area of Iran.[9] The part used of this plant and several other species of Ferula is an oleo-gum-resin [asafoetida] that obtained by incision of stem and root.[10] In Iranian folk medicine, asafoetida is considered as an anticonvulsant, diuretic, antispasmodic, antihelminthic, and carminative agent.[11] Recent pharmacological and biological studies have also shown several pharmacological activities such as antioxidant, anticonvulsant, antidiabetic, antispasmodic, and hypotensive.[11] It also demonstrated that this oleo-gum-resin has antileishmanial,[12] diuretic,[13] antinociceptive,[14] and aphrodisiac effects.[15] Asafoetida also has been reported to act as anticarcinogen in many traditional systems[16] which confirmed by a number of new studies.[11] A meta-analysis study showed that in countries such as Japan, Russia, China, and Indonesia, the rate of cancer is higher in the comparison of countries that usage of asafoetida is common.[17] These evidences provided reliable reasons for investigation of anticancer effect of asafoetida on viability 4T1 cells in vitro.
Materials and Methods
Essential oil and oleo-gum-resin preparation
F. assa-foetida oleo-gum-resin was collected from Tabas region (Yazd Province, Iran), and the plant species was botanically identified by Dr. Abbas Zarezadeh in Yazd Agricultural Research Center. The dried powder of asafoetida was soaked in distilled water overnight at room temperature and the yielded suspension was used.[18] Concentrations and dosages of the extract were expressed as crude amount of the dried oleo-gum-resin used in preparing the stock solution. For oil isolation of asafoetida, oleo-gum-resin (100 g) was dissolved in 1 L of distilled water and the oils were isolated by hydrodistillation using a Clevenger-type apparatus for 3 h. The distillated oils were dried over anhydrous sodium sulfate and stored at 4°C until used.
Cells and reagents
The 4T1 cells, which were purchased from the Pasteur Institute of Iran, were maintained in RPMI 1640 (Sigma-Aldrich) medium containing 10% (v/v) fetal bovine serum (Gibco Laboratories), 300 μg/ml glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin. The cell cultures were maintained at 37°C in a humidified atmosphere with 5% CO2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma, USA, and ferulic acid was purchased from Sigma, USA. Transwell plates for transwell migration assay were purchased from SPL Life Sciences Co. Ltd., Korea.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution
The MTTs were dissolved in sterile phosphate-buffered saline at 5 mg/ml and stored in dark condition at 4°C for a period lasting <3>
Cytotoxicity assay
Tumor cells in the exponential growth phase were harvested from culture flasks using 0.05% ethylenediaminetetraacetic acid (Gibco Laboratories) for 3 min. The cells were washed in standard growth medium and counted using a hemocytometer. A number of 3 × 103 cells per well were plated on 96-well flat-bottomed plates. One day after the seeding of the cells at 37°C, cells were treated for 24, 48, and 72 h with various concentrations of oleo-gum-resin and its essential oil and ferulic acid. Cells treated with serum-free medium for the same periods of time were used as a control. After that, the MTT solution (5 mg/ml in phosphate-buffered solution) was added to each well. After 3.5 h of incubation, purple crystals were formed by mitochondrial dehydrogenase enzyme of living cells. Then, the medium was discarded and 150 μl of dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance of each sample was read at 540 nm using a microplate reader (BioTek Instrument, Box 998). Results were expressed as percentage of cell viability with respect to untreated control cells (as 100%). The percent viability of each well was calculated from the following:

Statistical analyses
All data were expressed as means ± standard deviation. Graph Pad Prism version 5 (San Diego, California, USA) was used for data analysis. Statistically significant differences were determined using one-way ANOVA with Tukey–Kramer posttest for multiple comparisons. P < 0>
Results
Cytotoxicity effect of oleo-gum-resin on 4T1 cells
The oleo-gum-resin at 1–1000 μg/ml did not show any obvious cytotoxicity on breast cancer 4T1 cells in vitro after incubated for 24 h [Figure 1]. The inhibition effect of oleo-gum-resin on 4T1 cells was enhanced after incubated for 48 and 72 h. Cell viability was about 10% even at the highest concentration of 1000 μg/ml after 72 h.
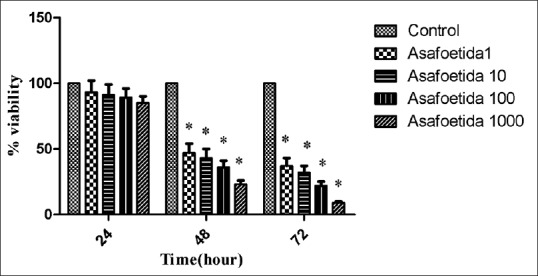
| Figure 1Cytotoxicity effect of asafoetida on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation
Effect of ferulic acid on 4T1 cells viability
Ferulic acid at 0.5–50 μg/ml did not show any obvious cytotoxicity on breast cancer 4T1 cells after incubated for 24, 48, or 72 h [Figure 2]. Although the inhibition effect of ferulic acid on 4T1 cells was enhanced after incubated for 72 h only at 500 μg/ml, the cell viability was about 20% even at the highest concentration of 500 μg/ml.
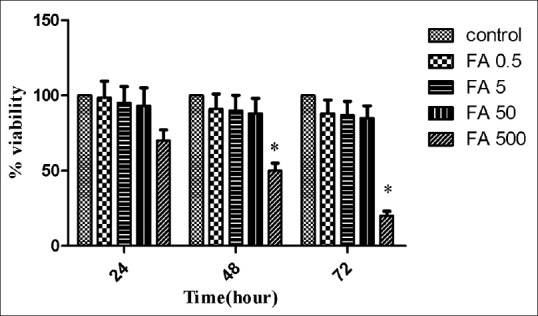
| Figure 2: Cytotoxicity effect of ferulic acid on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation
Effect of essential oil of asafoetida on 4T1 cells
Essential oil of asafoetida at 0.01 and 0.1 μl/ml did not show any obvious cytotoxicity on breast cancer cells after incubated for 24 h [Figure 3]. At doses of 1 and 10 μl/ml, a significant difference in viability percentage was observed in all the three times. The lowest dose of essential oil (0.01 μl/ml) could decrease significantly viability percentage only after 72 h. Furthermore, a significant difference was observed after 48 and 72 h at 0.1 μl/ml dose of essential oil.
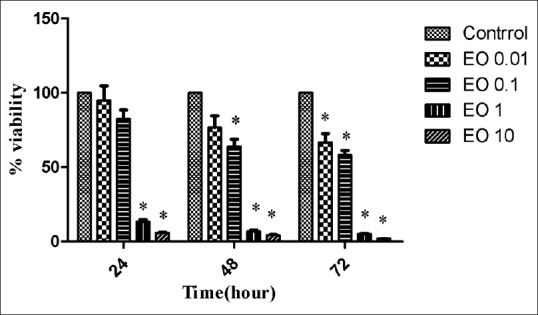
| Figure 3Effect of asafoetida essential oil on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation
Discussion
Throughout the world, Iranian medicinal F. assa-foetida has been widely consumed as health promoting, food supplements, and medicine. Given the increasing popularity promotion of F. assa-foetida for adjuvant cancer treatment, there is an interest to study the potential protection of F. assa-foetida against cancer, especially breast cancer cells. In the present study, we aimed to evaluate the antitumor effect of F. assa-foetida oleo-gum-resin, its essential oil, and ferulic acid, one of the main components of F. assa-foetida oleo-gum-resin, using the highly invasive mouse mammary carcinoma 4T1 cells. Several investigations demonstrated the cytotoxic activity of Ferula species. Bagheri et al.[10] determined the cytotoxicity of some Ferula species on Artemia salina as a model for evaluating general cytotoxicity. Hence, the species of Ferula could be as a good source of cancer chemopreventive agents. Asafoetida is dried latex exuded from the living rhizome, rootstock, or taproot of an umbelliferous plant of varied species.[11] Our results revealed that 48 and 72 h after incubation with asafoetida, cell viability was significantly decreased compared to control group that was dose- and time-dependent manner [Figure 1]. Our findings are consistent with the results of other researchers. In a study, it has been shown that asafoetida inhibits microsomal activation-dependent mutagenicity of 2-acetamidoflourene.[19] In spite of some old evidence about genotoxicity and mutagenicity of asafoetida, recent studies have revealed its potential antioxidant, antimutagenicity, and cancer chemopreventive activities.[11] In an in vivo study, Saleem et al. showed that pretreatment of animals with acetone extract of asafoetida could cause the reversal of early events of carcinogenesis.[16] Another study showed that asafoetida reduced the multiplicity and size of palpable mammary tumors in Sprague–Dawley rats.[11] Different mechanisms seem to impact on this activity such as radical scavenging activity and lipoxygenase inhibitory activity.[20] In the previous study, we showed that asafoetida has remarkable antioxidant and lipoxygenase inhibitory activity.[21] In addition, there are a number of biological activities in asafoetida that have been reported for this compound including cancer chemoprevention and apoptosis induction in cancer cells. Phytochemical analysis showed that asafoetida contains about 40%–64% resin, 25% endogenous gum, 10%–17% volatile oil, and 1.5%–10% ash. The resin portion is known to contain farnesiferol, asareninotannols, ferulic acid, umbelliferone, the gum includes glucose, galactose, l-arabinose, rhamnose, glucuronic acid, polysaccharides, and glycoproteins, and the volatile fraction contains sulfur-containing compounds, monoterpenes, and other volatile terpenoids.[11] Umbelliprenin is one of these components that has been shown to have remarkable cancer chemoprevention in vitro and in vivo.[22] Moreover, it was reported recently that farnesiferol C[23] from F. assa-foetida may be a potential candidate for the treatment of cancer. Phenolic compounds present in asafoetida have been reported to have strong antioxidants and antitumor properties.[24] In this study, we also investigated anticancer effect of ferulic acid which is one of the main phenolic constituents in asafoetida. Results showed that ferulic acid has anticancer at a dose of 500 μl [Figure 2]. The previous study showed that it has anticancer effect by increasing the effect of radiation treatment on human cervical carcinoma cells[25] and also has a synergistic role to inhibit cancer cell proliferation.[23] Among the components were tested, the essential oil has strongest cytotoxicity effect on 4T1 cells at the higher concentrations of 1 and 10 μg/ml [Figure 3]. Previously, researchers showed that this essential oil contains sulfur components and it is observed that E-1-propenyl sec-butyl disulfide (40.15%) and Z-1-propenyl sec-butyl disulfide (23.93%) were the major constituents of the oil.[26]
However, we observed that sulfur compounds of asafoetida have stronger anticancer effects, but we do not know the mechanism of these effects. However, some evidence suggests that organosulfur compounds modulate the activity of several metabolizing enzymes that activate (cytochrome P450s) or detoxify (glutathione S-transferases) carcinogens and inhibit the formation of DNA adducts in several target tissues.[27]
Conclusions
Our results demonstrated that asafoetida has a cytotoxic activity against mouse breast 4T1 cells. It seems that asafoetida has a set of natural compounds that have anticancer effects. In this study, it was found that the asafoetida oil has more potential to suppress cancer cells. However, its active components and their mechanism of actions need to be elucidated by further studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was supported by the foundation of Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
References
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-30.
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50.
- Yeap SK, Abu N, Mohamad NE, Beh BK, Ho WY, Ebrahimi S, et al. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement Altern Med 2015;15:306.
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005;55:178-94.
- Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci 2012;9:193-9.
- Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011;2011:941876.
- Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 2001;7:542-7.
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci 2005;78:431-41.
- Bagheri S, Hejazian SH, Dashti-R M. The relaxant effect of seed's essential oil and oleo-gum-resin of Ferula assa-foetida on isolated rat's ileum. Ann Med Health Sci Res 2014;4:238-41.
- Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol 2010;48:242-6.
- Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin) – A review. J Ethnopharmacol 2011;134:1-10.
- Bafghi AF, Bagheri SM, Hejazian SH. Antileishmanial activity of Ferula assa-foetida oleo gum resin against Leishmania major: Anin vitro study. J Ayurveda Integr Med 2014;5:223-6.
- Bagheri SM, Mohammadsadeghi H, Dashti-R MH, Mousavian SM, Aghaei ZA. Effect of Ferula assa-foetida oleo-gum-resin on renal function in normal Wistar rats. Indian J Nephrol 2016;26:419-422.
- ;Bagheri SM, Dashti-R MH, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci 2014;9:207-12.
- Bagheri SM, Yadegari M, Porentezari M, Mirjalili A, Hasanpor A, Dashti RM, et al. Effect of Ferula assa-foetida oleo gum resin on spermatic parameters and testicular histopathology in male wistar rats. J Ayurveda Integr Med 2015;6:175-80.
- Saleem M, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: A chemopreventive study. Life Sci 2001;68:1913-21.
- Nigam U, Sachan S. Evaluation of Ferula asafoetida for its anticancerous activity in different countries. J Pharmacogn Phytochem 2013;2:74-6.
- ;Azizian H, Rezvani ME, Esmaeilidehaj M, Bagheri SM. Anti-obesity, fat lowering and liver steatosis protective effects of Ferula asafoetida gum in type 2 diabetic rats: Possible involvement of leptin. Iran J Diabetes Obes 2012;4:120-6.
- Kochhar KP. Dietary spices in health and diseases: I. Indian J Physiol Pharmacol 2008;52:106-22.
- Mallikarjuna GU, Dhanalakshmi S, Raisuddin S, Rao AR. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast Cancer Res Treat 2003;81:1-10.
- Bagheri SM, Hedesh ST, Mirjalili A, Dashti-R MH. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa foetida oleo gum resin. J Evid Based Complementary Altern Med 2016;21:271-6.
- Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev 2009;18:412-5.
- Choi YE, Park E. Ferulic acid in combination with PARP inhibitor sensitizes breast cancer cells as chemotherapeutic strategy. Biochem Biophys Res Commun 2015;458:520-4.
- Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem 2002;50:5844-9.
- Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro 2011;25:1366-75.
- Lu TH, Mirzaei HH, Salehi M, Nekuei MK, Majidi E. Determination of Essential Oils, Antioxidants and Antimicrobial Effects of Ferula assa-foetid from Different Area of Iran; 2009. Available from: http://www.agris.fao.org/agris-search/search.do?recordID=IR2011000046.
- Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J 2010;18:51-8.

| Figure 1Cytotoxicity effect of asafoetida on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation

| Figure 2Cytotoxicity effect of ferulic acid on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation

| Figure 3Effect of asafoetida essential oil on 4T1 cells after 24, 48, or 72 h incubation. Data were expressed as mean ± standard deviation
References
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-30.
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50.
- Yeap SK, Abu N, Mohamad NE, Beh BK, Ho WY, Ebrahimi S, et al. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement Altern Med 2015;15:306.
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005;55:178-94.
- Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci 2012;9:193-9.
- Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011;2011:941876.
- Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 2001;7:542-7.
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci 2005;78:431-41.
- Bagheri S, Hejazian SH, Dashti-R M. The relaxant effect of seed's essential oil and oleo-gum-resin of Ferula assa-foetida on isolated rat's ileum. Ann Med Health Sci Res 2014;4:238-41.
- Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol 2010;48:242-6.
- Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin) – A review. J Ethnopharmacol 2011;134:1-10.
- Bafghi AF, Bagheri SM, Hejazian SH. Antileishmanial activity of Ferula assa-foetida oleo gum resin against Leishmania major: Anin vitro study. J Ayurveda Integr Med 2014;5:223-6.
- Bagheri SM, Mohammadsadeghi H, Dashti-R MH, Mousavian SM, Aghaei ZA. Effect of Ferula assa-foetida oleo-gum-resin on renal function in normal Wistar rats. Indian J Nephrol 2016;26:419-422.
- ;Bagheri SM, Dashti-R MH, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci 2014;9:207-12.
- Bagheri SM, Yadegari M, Porentezari M, Mirjalili A, Hasanpor A, Dashti RM, et al. Effect of Ferula assa-foetida oleo gum resin on spermatic parameters and testicular histopathology in male wistar rats. J Ayurveda Integr Med 2015;6:175-80.
- Saleem M, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: A chemopreventive study. Life Sci 2001;68:1913-21.
- Nigam U, Sachan S. Evaluation of Ferula asafoetida for its anticancerous activity in different countries. J Pharmacogn Phytochem 2013;2:74-6.
- ;Azizian H, Rezvani ME, Esmaeilidehaj M, Bagheri SM. Anti-obesity, fat lowering and liver steatosis protective effects of Ferula asafoetida gum in type 2 diabetic rats: Possible involvement of leptin. Iran J Diabetes Obes 2012;4:120-6.
- Kochhar KP. Dietary spices in health and diseases: I. Indian J Physiol Pharmacol 2008;52:106-22.
- Mallikarjuna GU, Dhanalakshmi S, Raisuddin S, Rao AR. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast Cancer Res Treat 2003;81:1-10.
- Bagheri SM, Hedesh ST, Mirjalili A, Dashti-R MH. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa foetida oleo gum resin. J Evid Based Complementary Altern Med 2016;21:271-6.
- Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev 2009;18:412-5.
- Choi YE, Park E. Ferulic acid in combination with PARP inhibitor sensitizes breast cancer cells as chemotherapeutic strategy. Biochem Biophys Res Commun 2015;458:520-4.
- Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem 2002;50:5844-9.
- Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro 2011;25:1366-75.
- Lu TH, Mirzaei HH, Salehi M, Nekuei MK, Majidi E. Determination of Essential Oils, Antioxidants and Antimicrobial Effects of Ferula assa-foetid from Different Area of Iran; 2009. Available from: http://www.agris.fao.org/agris-search/search.do?recordID=IR2011000046.
- Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J 2010;18:51-8.


 PDF
PDF  Views
Views  Share
Share

