Evaluation of micronutrient status in serum and saliva of oral submucous fibrosis patients: A clinicopathological study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(04): 224-226
DOI: DOI: 10.4103/0971-5851.107087
Abstract
Background: Oral submucous fibrosis (OSMF) is one of the most commonly occurring potentially malignant disorders in India and south East Asian countries where betel chewing is common practice. Iron and ascorbic acid are important agents for collagen synthesis. Aims: The aims of this study were to estimate the levels of iron and ascorbic acid in serum and saliva in patients with OSMF and to correlate change in levels of iron and ascorbic acid with the histopathological grading of OSMF. Materials and Methods: The study group comprised of 65 clinically diagnosed and histopathologically confirmed cases of OSMF; 21 age- and sex-matched controls were also enrolled in the study. Serum and salivary ascorbic acid were analyzed by the dintrophenyl hydrazine method whereas serum and salivary iron were analyzed by the dipyridyl method. Results: The serum and salivary ascorbic acid levels consistently decreased with the progression of histopathologiocal grading of OSMF. Serum and salivary iron levels were also decreased in OSMF patients, but this was not significant. Conclusion: Ascorbic acid and iron may have been used for the excessive collagen synthesis occurring during progression of OSMF. Hence, serum and salivary monitoring may play a crucial role in the early diagnosis and prognosis of OSMF.
Publication History
Article published online:
20 July 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Oral submucous fibrosis (OSMF) is one of the most commonly occurring potentially malignant disorders in India and south East Asian countries where betel chewing is common practice. Iron and ascorbic acid are important agents for collagen synthesis.
Aims:
The aims of this study were to estimate the levels of iron and ascorbic acid in serum and saliva in patients with OSMF and to correlate change in levels of iron and ascorbic acid with the histopathological grading of OSMF.
Materials and Methods:
The study group comprised of 65 clinically diagnosed and histopathologically confirmed cases of OSMF; 21 age- and sex-matched controls were also enrolled in the study. Serum and salivary ascorbic acid were analyzed by the dintrophenyl hydrazine method whereas serum and salivary iron were analyzed by the dipyridyl method.
Results:
The serum and salivary ascorbic acid levels consistently decreased with the progression of histopathologiocal grading of OSMF. Serum and salivary iron levels were also decreased in OSMF patients, but this was not significant.
Conclusion:
Ascorbic acid and iron may have been used for the excessive collagen synthesis occurring during progression of OSMF. Hence, serum and salivary monitoring may play a crucial role in the early diagnosis and prognosis of OSMF.
INTRODUCTION
Iron is one of the most abundant and necessary transition metals in the body, which is an essential component in DNA synthesis and in respiratory and oxidative metabolism.[1] Recent studies have revealed depleted iron levels in patients with oral pre-cancerous lesions.[2,3]
Ascorbic acid, or vitamin C, has the potential to protect both cytosolic and membrane components of cells from oxidant damage. In the cytosol, ascorbate acts as a primary antioxidant to scavenge free radical species that are generated as by-products of cellular metabolism.[4] Studies have revealed decrease in the ascorbic acid levels in oral submucous fibrosis (OSMF).[5]
OSMF is a fibrotic condition of the oral cavity and is always associated with chronic epithelial inflammation and progressive deposition of collagenous extracellular matrix (ECM) proteins in the subepithelial layer of the buccal mucosa.[6] The disease is seen in those from Indian subcontinent and from many parts of South-East Asia such as Taiwan.[7] We carried out a study with an objective of assessing the status of serum and saliva in different histopathological grades of OSMF.
MATERIALS AND METHODS
Eighty-six patients between the age range of 20 and 40 years, reporting to the Department of Oral Medicine and Radiology in a dental college in south India, were enrolled into the study. The study subjects included 65 histopathologically confirmed cases of OSMF and 21 age- and sex-matched healthy controls. A detailed case history that included habit index was taken from each subject in the study. Subjects with any other long-term systemic illness and long-term medication were excluded from the study. Five milliliters of unstimulated saliva was obtained by the spit method after following a standard pre-collection protocol. Five milliliters of venous blood was obtained from the antecubital vein, centrifuged and stored. Serum and salivary ascorbic acid were analyzed by the dintrophenyl hydrazine method whereas serum and salivary iron were analyzed by the dipyridyl method. The data obtained was subjected to statistical analysis using the SPSS version 17 software.
RESULTS
The OSMF subjects were graded histopathologically into three categories. Of the 65 subjects in the control group, 22, 20 and 23 were categorized under grade 1, 2 and 3, respectively [Figure [Figure1a1a–c] after histopathological examination. Mean serum and salivary ascorbic acid levels were significantly decreased in cases when compared with controls (P < 0.001). However, serum and salivary iron levels showed no significant difference in case and controls (P=0.073 and 0.097, respectively) [Table 1]. Both serum and salivary ascorbic levels consistently decreased as the histopathological grading progressed; there was a very highly significant difference among the three groups. Serum and salivary iron levels decreased as the grades progressed, but this was not significant [Table 2]. Serum and salivary levels showed significant correlation among cases (r=0.315 and P=0.011), but not among controls. Serum and salivary ascorbic acid levels showed no correlation among cases and controls [Tables 3 and 4]. There was no significant difference in the frequency of chewing habits and the levels of serum and salivary ascorbic acid or iron levels.
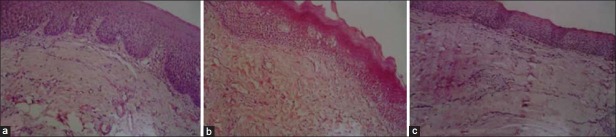
| Fig. 1 (a) Photomicrograph (40×) showing loose thin and thick fibers; (b) photomicrograph (40×) showing loose thin or thick fibers with partial hyalinization; (c) photomicrograph (40×) showing complete hyalinization
Table 1
Comparison between iron and ascorbic acid levels in cases and controls
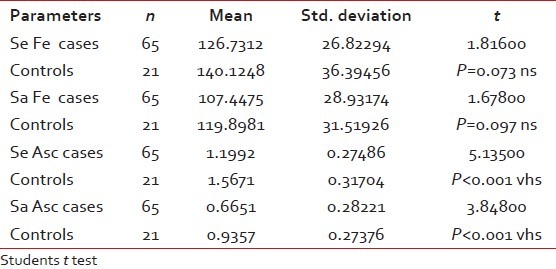
Table 2
Intercomparison of iron and ascorbic acid levels among histopathological grades of OSMF and controls
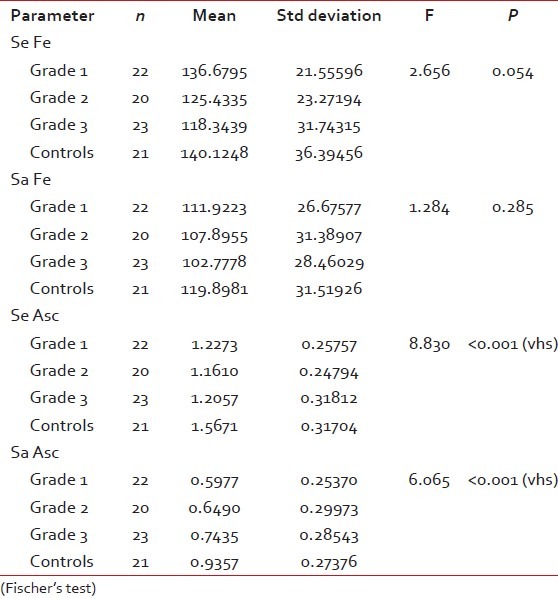
Table 3
Correlation between serum and salivary iron levels

Table 4
Correlation between serum and salivary ascorbic acid levels

DISCUSSION
The incidence of OSMF is increasing like an epidemic among youngsters in the Indian and South East Asian population. The etiology for OSMF is still obscure and a varied number of factors have been proposed, acrecanut chewing being the most important.[8] Several recent studies have been carried out on micronutrient status in OSMF; the copper and zinc studies being the most highlighted.[8–10] Ascorbic acid levels have been investigated in several cancer-related studies[11]; some studies have reported that ascorbic acid enhances destruction of cancer cells.[12] We observed a reduction in iron levels in OSMF cases, although it was statistically not significant. This decrease in iron levels could be due to the requirement of iron for collagen synthesis by enzymes in hydroxylation of proline and lysine. This hydroxylation of proline and lysine is catalyzed by proline hydroxylase and peptidyl lysine hydroxylase, respectively. Peptidyl proline hydroxylase requires as co-factors molecular oxygen, ferrous iron, alfa keto-glutarate and ascorbic acid.[13] We found a significant decrease in the levels of ascorbic acid in both serum and saliva. This could be attributed to its role in collagen synthesis. In a recent study involving 36 OSMF patients, tissue collagen and serum iron levels were assessed.[5] They found elevated tissue collagen levels and depleted ascorbic acid and iron reserves in OSMF patients. We used the dintrophenyl hydrazine method to assess serum and salivary ascorbic acid. This method was first used to assess salivary ascorbic acid in 1969.[14] Few studies however have stated that there is lack of correlation between serum and salivary ascorbic acid levels.[15] In our study, serum and salivary ascorbic acid levels showed good correlation. The ascorbic acid levels consistently increased as the histopathological grades of OSMF progressed. This indicated that iron and ascorbic acid may have been used for excessive collagen production and cross-linking occurring in OSMF. Hence, serum and salivary iron and ascorbic acid levels could be an important indicator for the progression of OSMF.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 (a) Photomicrograph (40×) showing loose thin and thick fibers; (b) photomicrograph (40×) showing loose thin or thick fibers with partial hyalinization; (c) photomicrograph (40×) showing complete hyalinization


 PDF
PDF  Views
Views  Share
Share

