Evaluation of myeloid cells (tumor-associated tissue eosinophils and mast cells) infiltration in different grades of oral squamous cell carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 158-167
DOI: DOI: 10.4103/0971-5851.190349
Abstract
Background: The multifunctional involvement and infiltration of myeloid cells (tumor-associated tissue eosinophils [TATE] and mast cells) can provide a unique opportunity to define relevant effectors functions that may represent novel, therapeutic options for modulation of tumor onset/growth. Aim: Our study aimed to evaluate infiltration of myeloid cells (TATE and Mast cells) infiltration in different grades (WHO grading) of oral squamous cell carcinoma (OSCC). Materials and Methods: Total 30 cases of OSCC were selected for this study. Hematoxylin and eosin stain and toluidine blue special stain, to evaluate TATE and the mast cells infiltration, were used. Three-year follow-up of OSCC cases was done. Result: Among 30 cases, 63.33% cases of OSCC showed TATE-positive and 36.66% cases showed TATE-negative. Regarding mast cells infiltration, 66.66% OSCC cases showed mast cells positive and 33.33% cases did not show significant mast cells infiltration. We found significant association of TATE and mast cells infiltration in OSCC cases. These myeloid cells infiltration significantly associated with age of patients but did not show any significant association with gender, site, % cases of OSCC showed TATE-positive and 36.66% cases showed TATE-negative. Regarding mast cells infiltration, 66.66% OSCC cases showed mast cells positive and 33.33% cases did not show significant mast cells infiltration. We found significant association of TATE and mast cells infiltration in OSCC cases. These myeloid cells infiltration significantly associated with age of patients but did not show any significant association with gender, site, and habit of cases. When we compared these cells infiltration with clinical stages and different histological grades of tumor, we found their infiltration is decreasing, from Stages 1 to Stage 3 of tumor and from well to poorly differentiated carcinoma. We have also found the less infiltration of these myeloid in recurrence cases of OSCC. Conclusion: As the infiltration of TATE and mast cells are correlated, along with evaluation of TATE, we should also evaluate the presence of mast cells infiltration in OSCC. The assessment of myeloid cells could become, in the future, useful for therapeutic approaches in this subset of the patient.
Keywords
Mast cells - oral squamous cell carcinoma - tumor-associated tissue eosinophils - toluidine bluePublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The multifunctional involvement and infiltration of myeloid cells (tumor-associated tissue eosinophils [TATE] and mast cells) can provide a unique opportunity to define relevant effectors functions that may represent novel, therapeutic options for modulation of tumor onset/growth.
Aim:
Our study aimed to evaluate infiltration of myeloid cells (TATE and Mast cells) infiltration in different grades (WHO grading) of oral squamous cell carcinoma (OSCC).
Materials and Methods:
Total 30 cases of OSCC were selected for this study. Hematoxylin and eosin stain and toluidine blue special stain, to evaluate TATE and the mast cells infiltration, were used. Three-year follow-up of OSCC cases was done.
Result:
Among 30 cases, 63.33% cases of OSCC showed TATE-positive and 36.66% cases showed TATE-negative. Regarding mast cells infiltration, 66.66% OSCC cases showed mast cells positive and 33.33% cases did not show significant mast cells infiltration. We found significant association of TATE and mast cells infiltration in OSCC cases. These myeloid cells infiltration significantly associated with age of patients but did not show any significant association with gender, site, and habit of cases. When we compared these cells infiltration with clinical stages and different histological grades of tumor, we found their infiltration is decreasing, from Stages 1 to Stage 3 of tumor and from well to poorly differentiated carcinoma. We have also found the less infiltration of these myeloid in recurrence cases of OSCC.
Conclusion:
As the infiltration of TATE and mast cells are correlated, along with evaluation of TATE, we should also evaluate the presence of mast cells infiltration in OSCC. The assessment of myeloid cells could become, in the future, useful for therapeutic approaches in this subset of the patient.
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is one of the leading causes of death in India. Sixty percent of oral cancers are well advanced by the time they are detected, and despite innovation being made in surgery, radiation, and chemotherapy, the long-term survival rate remains to be < 50%.[1] OSCC is malignant neoplasm arising from mucosal epithelium of oral cavity. It consists of heterogeneous cell population with different biologic characters.[2] The tumor microenvironment is a dynamic network that includes the cancer cells, stromal tissue (immune cells, fibroblast, myofibroblast, cytokines, and vascular tissue), as well as the extracellular matrix (ECM) that surrounds it all.[3] The immune system can respond to cancer cells in two ways: By reacting against tumor-specific antigens (molecule that is unique to cancer cells) or against tumor-associated antigens (molecules that are expressed differently by cancer cells and normal cells).[4] Cells of immune system comprised lymphoid series and myeloid progenitor series cells.[5] Myeloid cells are derived from hematopoietic stem cells and the types that are seen in human tumors include macrophages, hemangiocytes, and dendritic cells, as well as neutrophils, eosinophils, mast cells, and myeloid-derived suppressor cells.[6]
Tissue eosinophils are derived in hemopoiesis from CD34+ myeloid progenitors found in the bone marrow. The factors that influence the proliferation and the differentiation of the eosinophil lineage are cytokine growth factors including interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor and IL-5, which are important in promoting eosinophil differentiation. It is now well recognized that IL-5 is the key cytokine in terminal differentiation of eosinophil from committed precursors.[7,8] Eosinophils are granule containing cells that are 8 µm in diameter, and their nuclei are usually bilobed although three or more lobes are often observed. The eosinophils are characterized by its bright red granules with the dye such as eosin under light microscope, while under electron microscope, this granule shows electron-dense crystalloid core surrounded by less electron dense granule matrix.[7,9] Other cell of interest in myeloid group is mast cell. It is round or elongated in shape and can be appreciated as large cells with diameter varying from 5 to 25 μm. The nucleus is ovoid and nonsegmented, and in the cytoplasm, there are the usual cell organelles, such as the Golgi apparatus, mitochondria, and some endoplasmic reticulum. However, the dominant cytoplasmic element is granules.[10,11] Mast cells are derived from multipotential stem cells in bone marrow.[12,13] Mast cells originate from the bone marrow as immature cells and migrate to peripheral tissues where they mature in situ. Mast cells are now recognized as an early and persistent infiltrating cell type in many tumors, often entering before significant tumor growth. Mast cells accumulate at the boundary between healthy tissues and malignancies and are often found in close association with blood vessels within the tumor microenvironment.[14]
Eosinophil produce nerve growth factor (NGF),[15] a cytokine not only involved in survival and functional maintenance of sympathetic neurons but also in immune regulation. NGF acts in an autocrine fashion by activating release of eosinophil peroxidase (EPO). NGF also promotes mast cell survival and activation.[15,16] Thus, eosinophils have the capacity to regulate mast cell function. Moreover, eosinophils are also thought to become active following the action of mast cells, as this cells secret histamine and eosinophils chemoattractant factor (ECF) which attract eosinophils in tissue.[17] Mast cells are larger than eosinophils, and these are multifunctional cells which play a central role in acquired and innate immunity as well as in allergic inflammation. Mast cells are local resident of connective tissue.[10] The most striking morphological feature of mast cells is the larger number of strongly metachromatic granules present in the cytoplasm.[17] It was not until the early 1980s that study of tissue eosinophilia in head and neck cancer gained attention. In the majority of the reports, tumor-associated tissue eosinophilia correlated with favorable outcomes. Nevertheless, unfavorable association has also been reported. Tumor-associated tissue eosinophils represent a local inflammatory reaction leading to tumor cell damage. Furthermore, detection of tumor necrosis factor-alpha (TNF-α) suggested that tumor-associated tissue eosinophils may play a role in the host defense mechanism. However, the actual role of tumor-associated eosinophil on tumor stroma remained a controversial topic.[18] Mast cell mediators are known to affect endothelial cells by inducing vasodilation. They also help in recruitment of inflammatory cells. It has been postulated that mast cells play a role in promoting angiogenesis in some malignant tumors. However, in some studies, high mast cell density has been found to correlate with favorable prognosis whereas other studies show a negative correlation. Thus, a controversy still exists.[18] Till now, there are only few literature [18,19,20] in which both myeloid cells (Tumor-associated tissue eosinophils [TATE] and mast cells) has been observed in the OSCC. Hence, in search of new predictive factors for OSCC, our study aimed to evaluate the significance of tissue eosinophil and mast cell infiltration in different grades (WHO grading) of OSCC.
MATERIALS AND METHODS
The study was carried out after obtaining approval from the Institutional Ethical Committee, DMIMS, Sawangi (M). It was retrospective study.
Inclusion criteria
-
Histopathologically diagnosed cases
-
Surgically operated OSCC cases
-
Intraoral primary tumor cases of OSCC.
Exclusion criteria
-
Immunocompromised cases such as the patients suffering from autoimmune diseases and HIV patients
-
Tumors with extensive ulceration and/or necrosis. The sample size of 30 histopathologically diagnosed cases of OSCC with 10 well, 10 moderate, and 10 poorly differentiated squamous cell carcinoma (PDSCC) was included in this study. Toluidine blue was used for mast cells staining and hematoxylin and eosin (H and E) stain was used for tumor-associated tissue eosinophil staining. Three-year follow-up of OSCC cases was done.
Common steps for staining methods
The study was performed on paraffin embedded tissue which was fixed in 10% neutral buffered formalin and routinely processed. Paraffin wax blocks were cut using LEICA soft tissue microtome, and the sections of 5 micron were used for staining. All sections were dewaxed thoroughly in xylene and hydrated through descending grades (100%, 90%, 80%, and 70%) of alcohol to water. For TATE staining, routine H and E stain was used, after staining eosinophil granules appeared as reddish to pink and nuclei take blue color. Special stain toluidine blue was used for mast cells staining. For this, 0.5% toluidine blue (0.5 g toluidine blue in 100 ml distilled water) solution was prepared and sections were stained in this solution for 30 s. After staining mast cells, granules appeared as red in color and rest tissue were appeared as varying shades of blue color. Common steps after staining-sections were washed in tap water then dehydrated through ascending grades (70%, 80%, 90%, and 100%) of alcohol, cleared and mounted in Distyrene plasticizer xylene.[17,21] Slides were examined under light microscope [Figures [Figures11 and and2].2]. High-density areas of infiltration of these myeloid cells were selected randomly in section and cells were counted in high power fields (HPFs). Scoring criteria: < 10 = 0, 10–30 = 1, 30–50 = 2, >50 = 3. For evaluation of the inter/intraexaminer consistency, slides were observed by two more examiners for counting of these cells.
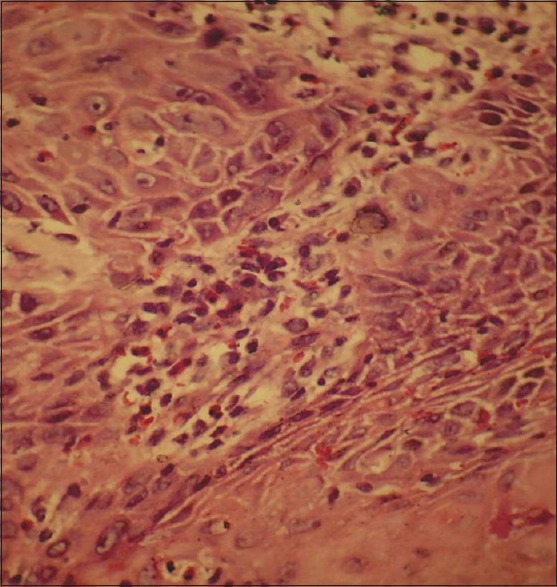
| Fig. 1 Section shows presence of tumor-associated tissue eosinophils in tumor stroma (H and E)
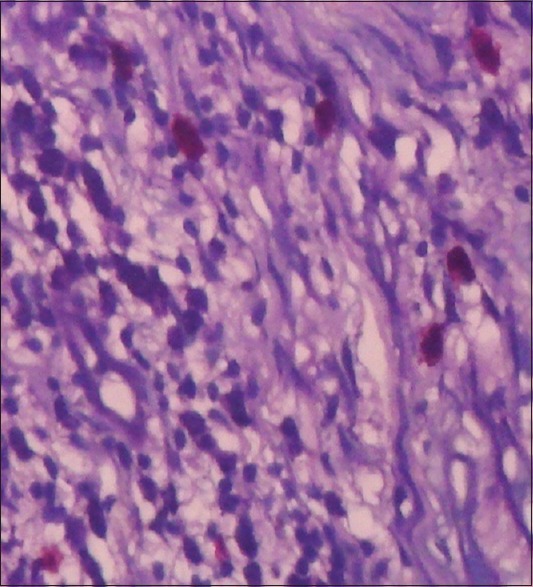
| Fig. 2 Toluidine blue stained section shows presence of mast cells (with red metachromatic granules)
RESULTS
The data were collected from all cases and organized in a systemic manner. All data were formulated in table and graph derived from statistical analysis, for interpretation of results.
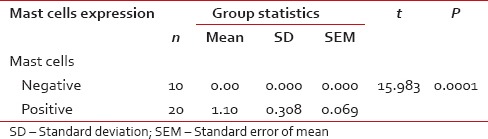
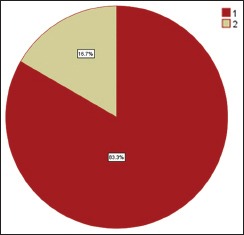
Among 30 cases, 63.33% cases of OSCC showed TATE-positive and 36.66% cases showed TATE-negative [Table 1]. Regarding mast cells infiltration, 66.66% OSCC cases showed mast cells positive and 33.33% cases did not show significant mast cells infiltration [Table 2]. Among our 30 cases of OSCC; 50% cases were T2N0M0, 30% T1N0M0, 13.3% T3N1M0, 3.3% T3N0M0,3.3%T2N1M0 [Graph 1]. These myeloid cells infiltration significantly associated with age of patients but did not show any significant association with gender, site, and habit of cases [Tables [Tables33 and and4].4]. We compared these cells infiltration with clinical stages of tumor, we found that their infiltration is decreasing, from Stages 1 to Stage 3 of tumor. TATE infiltration showed significant difference (P < 0.05) in clinical stage of tumor whereas mast cells infiltration did not show any significant difference [Tables
[Tables5,5, ,66 and Graphs 2, ,3].3]. When we compared infiltration of TATE and mast cells in different histological grades of tumor, from well to poorly differentiated carcinoma, we found that their mean score of infiltration is decreasing from well to PDSCC Tables Tables7,7, ,88 and Graphs Graphs4,4, ,5].5]. Three-year follow-up was done to see any recurrence cases. We also found the less infiltration of these myeloid cells in recurrence cases of OSCC [Table 9 and Graph 6]. We found significant association of TATE and mast cells infiltration in OSCC cases [Graph 7].Table 1
Infiltration of eosinophils in positive and negative cases of oral squamous cell carcinoma (mean and P value)
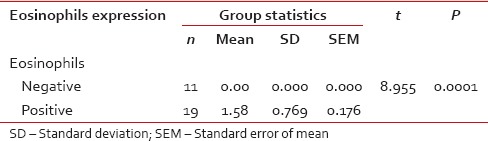
Table 2
Infiltration of mast cells in positive and negative cases of oral squamous cell carcinoma (mean and P value)
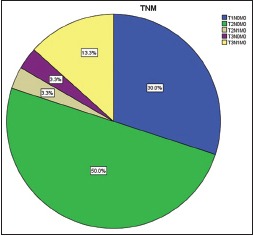
| Graph 1 Percentage of oral squamous cell carcinoma cases as per tumor node metastasis classification
Table 3
Comparison of clinical parameters with eosinophils infiltration
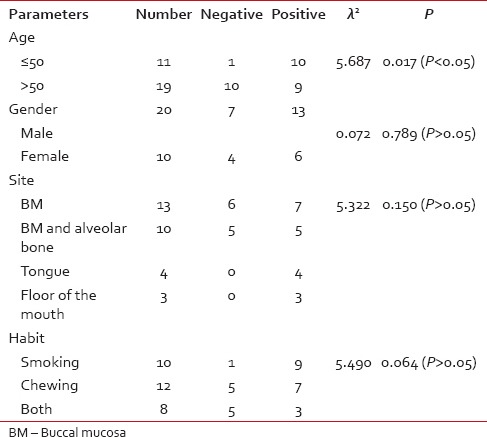
Table 4
Comparison of clinical parameters with mast cells infiltration
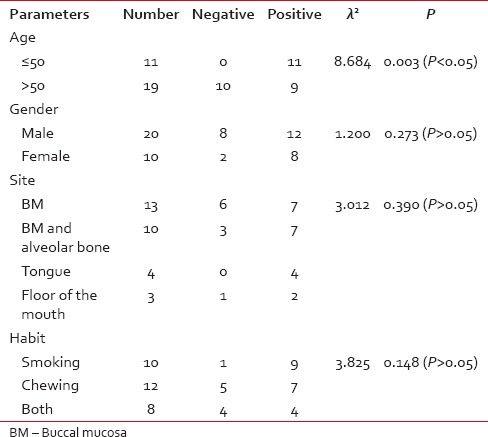
Table 5
Comparison of eosinophils infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)
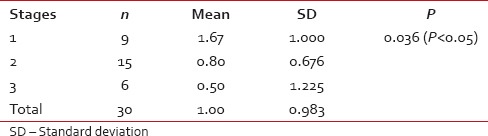
Table 6
Comparison of mast cells infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)
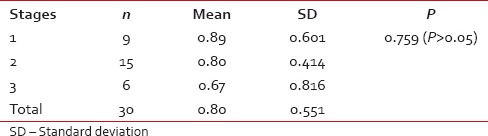
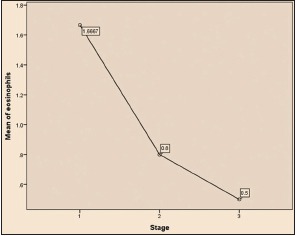
| Graph 2 Comparison of eosinophils infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)
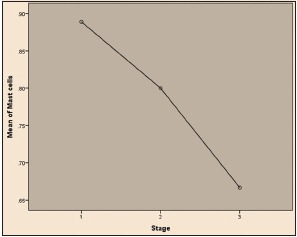
| Graph 3 Comparison of mast cells infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)
Table 7
Eosinophils infiltration in normal and in oral squamous cell carcinoma cases (ANOVA test)
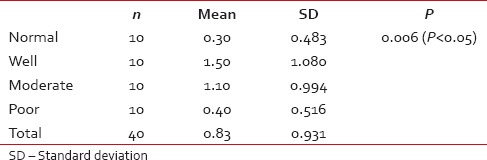
Table 8
Mast cells infiltration in normal and in oral squamous cell carcinoma cases (ANOVA test)
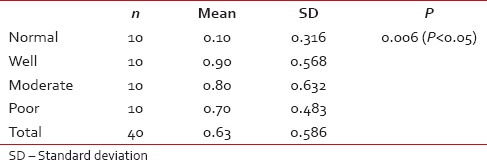
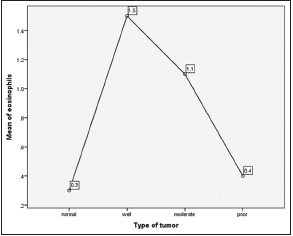
| Graph 4 Eosinophils infiltration in normal and in oral squamous cell carcinoma cases (ANOVA test)
| Graph 5 Recurrence and no recurrence cases of OSCC
Table 9
Eosinophils and mast cells expression in recurrence and no recurrence cases of oral squamous cell carcinoma
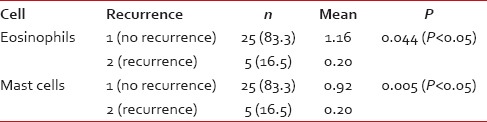
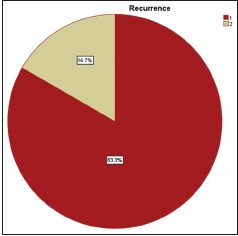
| Graph 6 Eosinophils and mast cells expression in recurrence and no recurrence cases of oral squamous cell carcinoma
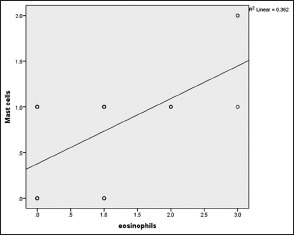
| Graph 7 Correlation of eosinophils & mast cells infiltration in OSCC. Correlation coefficient value r = 0.602 and P value = 0.000
DISCUSSION
The immunosurveillance hypothesis posits that the immune system recognizes malignant cells as foreign agents and eliminates them. There is the potential of immune system to control cancer and the various ways that immunotherapy can boost the potential of immune system for the benefit of the patient.[22] Lorena et al. did study to compare the number of eosinophils identified routinely with H and E stain and by immunohistochemistry in OSCCs with TATE and found that there was no statistically significant difference (P > 0.05) in the number of eosinophils/mm2 identified by H and E stain or immunostaining technique in OSCCs with TATE.[23] So in this study, routine H and E stain was used to evaluate the infiltration of TATE in different histological grade of OSCC. Mast cells are not readily identified in H and E stains because their metachromatic granules are refractile and do not take up the stain. This metachromasia is due to the high concentrations of the sulfated mucopolysaccharide heparin.[17] Hence, special stain toluidine blue was used for mast cells staining. Although mononuclear cells, and to a lesser extent neutrophils, are also found in oral cancers, eosinophils when present, from the predominant inflammatory cell population.[24,25,26] Among 30 cases, 63.33% cases of OSCC showed TATE-positive and 36.66% cases showed TATE-negative [Table 1]. Regarding mast cells infiltration, 66.66% OSCC cases showed mast cells positive and 33.33% cases did not show significant mast cells infiltration [Table 2]. There are numerous methods for evaluation of tissue eosinophil and mast cell and many investigators use different grading systems. Loe and Fletcher considered more than 10 eosinophils per HPF to be moderate TATE and more than 100 eosinophils per HPF to be massive TATE. Goldsmith et al. assessed the prominence of eosinophils within the inflammatory infiltrate on a scale of 0–4+, according to the following criteria: 0 equals no eosinophil; 1 + equals 5–10 eosinophils per HPF; 2 + equal 10–20 eosinophils per HPF; and 3 + equals more than 30 eosinophils per HPF. Sassler et al. assigned the criteria as low, medium, or high-grade classification (1–5, 5–10, or >10 eosinophils per HPF, respectively). Deron et al. declared a tumor to be TATE-positive if more than 2 eosinophils per HPF were found.[27] Horiuchi et al. selected the area with the highest number of tissue eosinophil/mast cells around the tumor and counted them in 10 HPF, chosen at random.[28] Alkabuli stated that if boundaries of the classical method were modified than we can achieve good correlation between classical method (in 10 HPF) and density method (with use of grid) and suggested modification in classical method as low (>10), moderate (50–120), and heavy (120 above) grades for eosinophils in per 10 HPF.[25] We found that in normal group, tissue eosinophils count ranged from 0 to 8 eosinophils per 10 HPF. Hence, we consider the tumors to be TATE-positive if count was ≥ 10 eosinophils per 10 HPF. A count of < 10 eosinophils per 10 HPF was considered as a TATE-negative (0). Scoring criteria: < 10 = 0, 10–30 = 1, 30–50 = 2, >50 = 3. For evaluation, high-density area was chosen at random. For mast cell evaluation, we have followed the same method as used for eosinophil counting. According to Igarashi et al., tissue eosinophilia has been found in 22–89% of all malignant tumors.[29] It has been observed that TATE and mast cells infiltration is significantly increased in OSCC patients in comparison to normal group of patients and the infiltration of these myeloid cells (TATE and Mast cells) is decreasing from well to poorly differentiated OSCCs and it was found that mean ± standard deviation value of TATE and mast cells/10 HPF is increased in WDSCC in comparison to moderately differentiated squamous cell carcinoma and PDSCC. The increase in number of eosinophil was also reflected with an increase of mast cell, as mast cell secrets ECF and histamine which attract tissue eosinophils.[17] We also found the less infiltration of these myeloid cells in recurrence cases of OSCC during their 3-year follow-up. Recurrence and regional lymph node metastases are two major hurdles in the management of the OSCC. Thus, a comprehensive investigation of the factors and molecular events which contribute to recurrence and invasion of OSCC are necessary for the development of novel strategies for prognostication and treatment. Regarding the antitumoral role of TATE in OSCC was also evaluated in several studies [19,30,31,32,33,34] done by several authors like Debta et al,[19] Lowe D.[30] Gold Smith MM et al,[31] Gold Smith MM et al,[32] Gao J et al[33] and Dorta RG et al.[34] All these studies in OSCC provided evidence for increased number of tissue eosinophil association with antitumoral role and good prognosis. Tumor-associated eosinophil has also been studied in various other malignancies of the body such as malignancies of colon, cervix, larynx, esophagus, and nasopharynx [35,36,37,38,39] suggested that tumor-associated tissue eosinophils are associated with favorable prognosis and this is indicative of good immune response of the body. However, it remains unknown whether it is the eosinophils themselves that lead to the improved prognosis or simply that tissue eosinophilia is a coincidental epiphenomenon initiated by a more fundamental biologic process. There is some experimental evidence for the former since it has been shown that the growth of implanted murine tumors is inhibited if the proposed implanted site has eosinophilia.[37] Direct damage to mammalian tumor cells by the eosinophil-mediated peroxidase system has also been demonstrated. TNF-α, secreted by eosinophils, also play an important role in OSCC as it causes death of tumor cells.[34]
The studies done by Tanooka et al., Sand et al., Samoszuk et al., Ch'ng et al., Alkhabuli, Sinnamon et al. and Ueda et al. have provided evidence for the association of increase number of mast cells with favorable prognosis and suggest that mast cells play a defensive and antitumoral role.[18,40,41,42,43,44,45] Mast cells are versatile in function and capable of regulating inflammation, host defense and innate immunity by elaboration of several chemokines and cytokines. Mast cell accumulation in tumor is probably part of a response to tumor-derived chemoattractant.[46,47] Antitumoral role of mast cells is explained by various mediators that are detrimental to the tumor including cytokines IL-1, IL-4, and IL-6 which induce apoptosis of tumor cells and chondroitin sulfate inhibit metastasis. Mast cells also produce TNF-α, is directly cytotoxic to tumor cells.[43,48] In experimental mice, it has been seen that mast cell deficient mice had an increased tumor incidence after treatment with a carcinogenic agent.[40] Thus all this evidence suggest that tumor-associated tissue mast cell play a role in antitumoral activity and thus show association with a favorable prognosis.
Tumors are complex tissues whose fate depends on the levels of pro-versus antitumorigenic signals that are provided by the tumor cells, by the local tumor microenvironment (including by resident and recruited immune cells), and by the host systemically. All of these processes can potentially be negatively or positively regulated by individual products released by inflammatory cells. It is correct to point out that mast cells population and tumors can exhibit heterogeneity of phenotype, defining mechanistically how mast cells interfere with or promote the survival and progression of particular types of tumors is likely to continue to represent a challenge.[49] We believe that the understanding of the precise role of mast cells in tumor microenvironment and its interaction with other inflammatory cells infiltration will be of critical importance for the development of new targeted therapies in human cancer.
The mast cells are an important source of several proangiogenic and angiogenic factors such as histamine, heparin, chymase, basic fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β).[50] Several proteases released by mast cells (MMP-9 and the serine proteases chymase and tryptase) are proangiogenic; they can degrade components of the ECM and contribute to tumor invasiveness.[51,52] Once secreted, mast cell mediators can do the following: (a) Initiate tissue and immunological responses; (b) attract inflammatory cells; and (c) mediate tissue remodeling and repair.[53,54,55,56] Differences in response lie in the ability of mast cells to secrete pro-inflammatory (mainly TNF-α) or anti-inflammatory (IL-10 and TGF-β) cytokines. For example, mast cells can secrete TNF-α and increase antigen presentation by dendritic cells, promoting pro-inflammatory T cell responses and monocyte/macrophage activation. However, under specific conditions, mast cells can secrete IL-10 and thus block T cell proliferation.[53,57] Moreover, mast cells can modulate adaptive immunity and angiogenesis [57,58] through the release of cytoplasmic granules and cytokines (mainly IL-1, TNF-α, and IL-6) and growth factors (VEGF, TGF-β, FGF, angiopoietin-1). Therefore, mast cells can modulate the intensity of organ injury depending on the pathophysiological context.[59] Ch'ng et al. stated in their experiment as like other inflammatory cells, mast cells are attracted to tumors by various factors including hypoxia, cellular damage, tissue ischemia and tumor-derived chemoattractants including stem cell factor, IL-3, and IL-4. They, in turn, produce various cytokines such as TNF-α, IL-1, IL-4, and IL-6 which can induce apoptosis of tumor cells, suggestive of an antitumoral role of mast cells. They concluded that mast cells have a direct inhibitory effect on the proliferation of mucosal squamous cell carcinoma.[43] Iamaroon et al. conducted a study on 26 OSCC patients to determine the correlation between the number of mast cells and microvessels densities. The densities of mast cells and microvessels increased in tumorigenesis which suggests that mast cells and microvessels may be used as indicator for tumor progression.[60] The cell-mediated cytotoxic effects of the mast cells have been reported, with mast cell: Tumor ratios which were >20:1. Conversely, the cytotoxic effects of the mast cells were nullified and the tumor progression was found to be enhanced when the mast cell-tumor ratios were increased from 10:1 to 1:100. Hence, the effect of mast cells against the cancer cells might depend on the concentration of the mast cells might depend on the concentration of the mast cell products in the microenvironment. Based on these findings, Tomita et al. hypothesized that reversing this process, i.e., enhancing the cytotoxic functions of the mast cells and suppressing their angiogenic functions, could lead to a new anticancer treatment strategy.[61]
A recent study done by Davoine et al. and published in 2013 suggest that eosinophils may contribute to the inflammatory response observed in OSCC and limit tumor progression by subsequent anti-tumor activity through the action of cationic proteins. They observed that inhibition of OSCC growth correlated with detectable cytotoxic granule enzyme EPO activity in culture medium.[62] Regardless, basic proteins from eosinophil granules are extremely cytotoxic, thus, small concentration of free exocytosed granules may be sufficient to exert a potent inflammatory/cytotoxic response against tumor cells.[63] In addition to these potential cytotoxic effector activities, eosinophils are also capable of exarting an immunoregulatory role in relation to the tumor environment. Eosinophils secrete a wide range of cytokines chemokines and growth factors and these may further contribute to the biological and immunological role of the eosinophil in OSCC.[64] Various causes for tumor-associated eosinophilia have been postulated: Tumor-inducing eosinophilia; tumor antigenicity-stimulated T lymphocytes that attract eosinophils by means of chemotactic factors; tumor antigens combining with antibodies to form immune complexes resulting in eosinophilia; and tumor secretagogues having eosinophil chemotactic ability or eosinophilopoietic activity. Confirmatory evidence has been sporadic, and it is likely that several causes are responsible.[65,66] Regardless of the cause of accumulation or the mechanism by which eosinophils traffic to tumors, a salient question remains: What are the consequences of this eosinophil infiltration? Specially, are eosinophils destructive, cytotoxic effector cells limiting tumor growth as part of a host surveillance mechanism, or do the infiltrating eosinophils facilitate tumor growth by remodeling and immunoregulation of the tumor microenvironment? Eosinophils can be related to cytokines of Th1 and Th2 response via synthesis and release of interferon-γ and IL-4, IL-5, and IL-10. In head and neck squamous cell carcinoma, it has been shown that the Th1 response is mainly associated with a better prognosis than those with the Th2 response.[67,68] Early stage of OSCC was found to express mainly INF-γ and IL-2 genes (Th1 responses) whereas the advanced stage tumors have IL-4 and IL-10 [removed]Th2 response).[69] Lorena et al. found intimate association of eosinophils with strong lymphoplasmacytic cell infiltration in OSCC.[23] Cormier et al. suggest that the recruitment and accumulation of eosinophils to tumors are part of a site-specific, early host-recognition response.[70] With better characterization of infiltrating immune cells, the precise role of inflammation in cancer has begun to be elucidated, leading to a resolution of the initial contradiction that inflammation is protective in certain tumors yet detrimental in others. The malignant state is unleashed by defect in communication pathways, which recruit host cells to become active participants. Inflammatory cells and their interaction with different cytokines have significant impact on carcinoma. Advances in diagnosis and treatment have slowly accumulated but a sound understanding of underlying cell biology is likely to enable future, much-needed progress. Although TATE and mast cells are commonly encountered in human cancer, their functional role in the tumor microenvironment remains an ambiguity, can represent another promising approach in cancer immunotherapy. Immunotherapy using IL-2 has been shown to have moderate success against some tumors and is often associated with “unexpected” but significant eosinophilia, which resulted in assumptions suggesting that eosinophils possess anti-tumor activity, at least in vitro.[71] IL-2 is recognized as a potent regulator of eosinophil activation, in vitro.[72,73] IL-2 induced release of EPO in culture media is associated with inhibition of oral cancer cell proliferation.[62] Myeloid cells represent novel targets for therapeutic strategies. The mobilization and recruitment of myeloid cells by the tumor defines myeloid cells as a potential delivery system to target the tumor microenvironment. Targeting cytokines and cytotoxic proteins to tumors by means of gene-modified myeloid cells thus represents a promising strategy to treat cancer.[74] Thus, we can suggest that the infiltration of TATE and mast cells (myeloid cells) could become, in the future, useful for therapeutic approaches in OSCC cases.
CONCLUSION
Now, it is time to integrate the quantification of these myeloid cells (TATE and mast cells) infiltration for routine microscopic evaluation of OSCC. Thus, careful studies on the nature of efficient immune reactions, the place where they are initiated, the cell and molecules involved, and their impact at different stages of the disease should provide new tool and goal for targeted therapies. Most pathologists do not routinely count eosinophils in malignant tumors although this determination in H and E sections can be easily performed. Furthermore, one should be attentive more if higher eosinophil counts are evident in OSCC. The eosinophil and mast cells counts could become, in the future, useful for therapeutic approaches in this subset of patients. Hence, it is recommended that quantitative assessment of eosinophils and mast cells should become part of the routine diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Section shows presence of tumor-associated tissue eosinophils in tumor stroma (H and E)

| Fig. 2 Toluidine blue stained section shows presence of mast cells (with red metachromatic granules)

| Graph 1 Percentage of oral squamous cell carcinoma cases as per tumor node metastasis classification

| Graph 2 Comparison of eosinophils infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)

| Graph 3 Comparison of mast cells infiltration with stages of oral squamous cell carcinoma tumor (ANOVA test)

| Graph 4 Eosinophils infiltration in normal and in oral squamous cell carcinoma cases (ANOVA test)

| Graph 5 Recurrence and no recurrence cases of OSCC

| Graph 6 Eosinophils and mast cells expression in recurrence and no recurrence cases of oral squamous cell carcinoma

| Graph 7 Correlation of eosinophils & mast cells infiltration in OSCC. Correlation coefficient value r = 0.602 and P value = 0.000


 PDF
PDF  Views
Views  Share
Share

