Ewing Sarcoma of Phalanx—A Common Tumor with an Uncommon Presentation
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(02): 189-192
DOI: DOI: 10.1055/s-0042-1742443
Introduction
Ewing sarcoma (ES) is a small, blue, round cell tumor usually affecting long bones. In 90% of the cases, it occurs in patients aged from 5 to 25 years. While the incidence shows a peak during the second decade of life, it is extremely rare after the third decade of life.[1] [2] The disease shows a slight predilection for males (male to female ratio of 1.6:1.0).[3] It rarely arises in fingers. Here, we present a case of ES of phalanx in an adult.
Source(s) of support
None
Declarations
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity but anonymity cannot be guaranteed.
Competing Interests
None of the authors have any competing interests.
Authors' Contributions
SB and AC contributed to the conception of the study.SV, LAJ, LD, and SS were responsible for the acquisition. SA, LAJ, SBMC, LKN, AHR, and LKR drafted the work. LD, LAJ, and LKR substantively revised it. All authors have read and approved the manuscript.
Publication History
Article published online:
14 March 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Introduction
Ewing sarcoma (ES) is a small, blue, round cell tumor usually affecting long bones. In 90%-of the cases, it occurs in patients aged from 5 to 25 years. While the incidence shows a peak during the second decade of life, it is extremely rare after the third decade of life.[1] [2] The disease shows a slight predilection for males (male to female ratio of 1.6:1.0).[3] It rarely arises in fingers. Here, we present a case of ES of phalanx in an adult.
Case Report
A 46-year-old woman presented to our hospital with a 10-month long history of local swelling and 5 months history of pain involving right middle finger. There was no history of fever, loss of weight, or trauma. The swelling was nearly 7 cm × 7 cm x 11 cm in dimensions, firm, tender, immobile with no local rise of temperature. The range of motion of the second, third, and fourth metacarpophalangeal joint was restricted ([Figs. 1] and [2]).
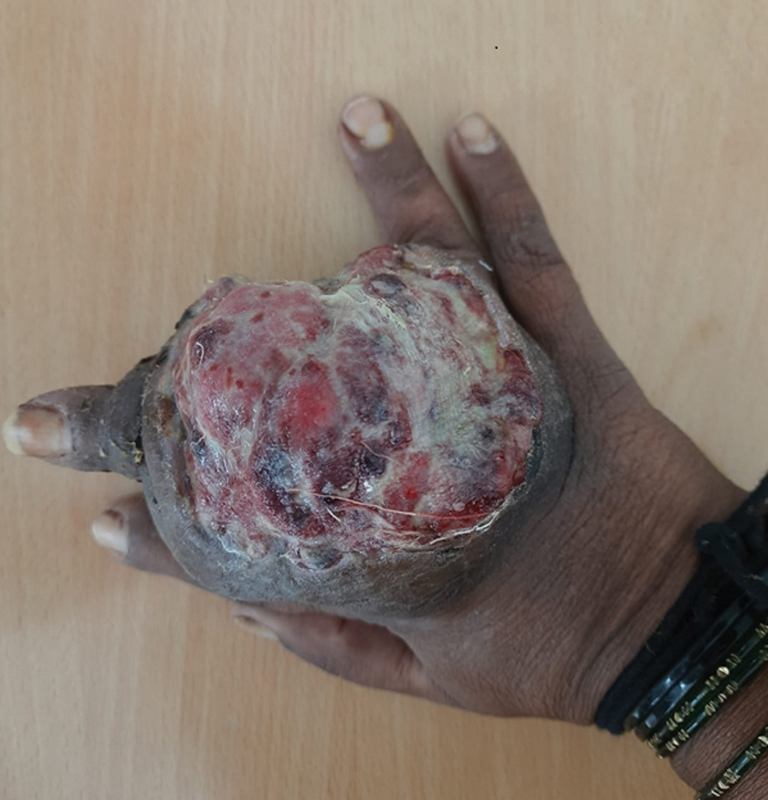
| Fig. 1Clinical photograph depicting swelling involving the third phalanx on dorsal surface of hand.
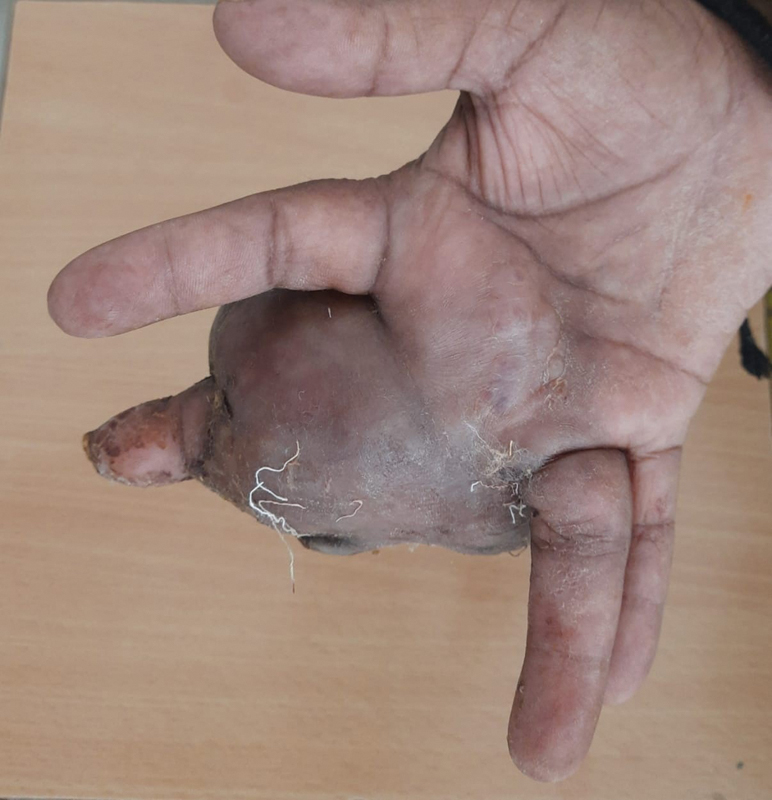
| Fig.2:Clinical photograph ventral surface of hand showing swelling involving third phalanx.
X-ray of the right-hand revealed bone destruction along with sclerosis of proximal phalanx and involvement of soft tissue component ([Fig. 3]). Laboratory findings including complete blood counts and biochemistry were within normal limits except for the levels of serum lactate dehydrogenase that was elevated to almost three times the normal. Magnetic resonance imaging (MRI) of right hand revealed altered signal intensity in soft tissue lesion circumferential to third metacarpal, proximal, and middle phalanx with areas of cortical breach. Bone marrow examination showed that it was not involved. The histopathological examination revealed dense cellularity with small, round cells uniformly arranged in sheets along with areas of necrosis ([Fig. 4]).
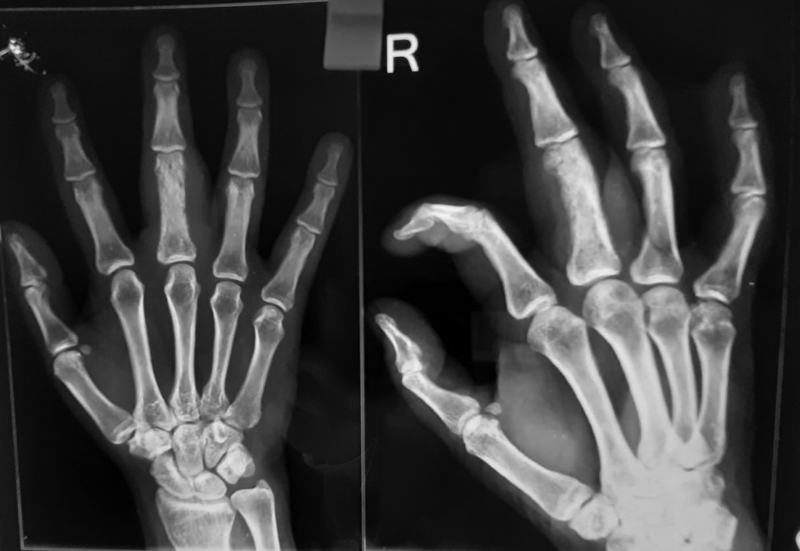
| Fig.3:Radiograph revealing bone destruction with sclerosis of proximal phalanx of middle finger and involvement of soft tissue component.
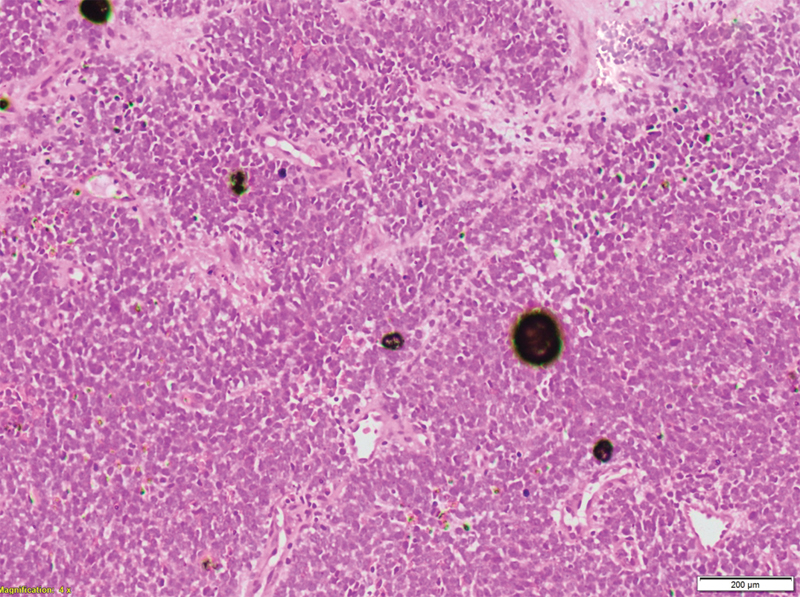
| Figure.4:Hematoxylin and eosin staining showing dense cellularity with small round blue cells uniformly arranged in sheets along with few areas of necrose.
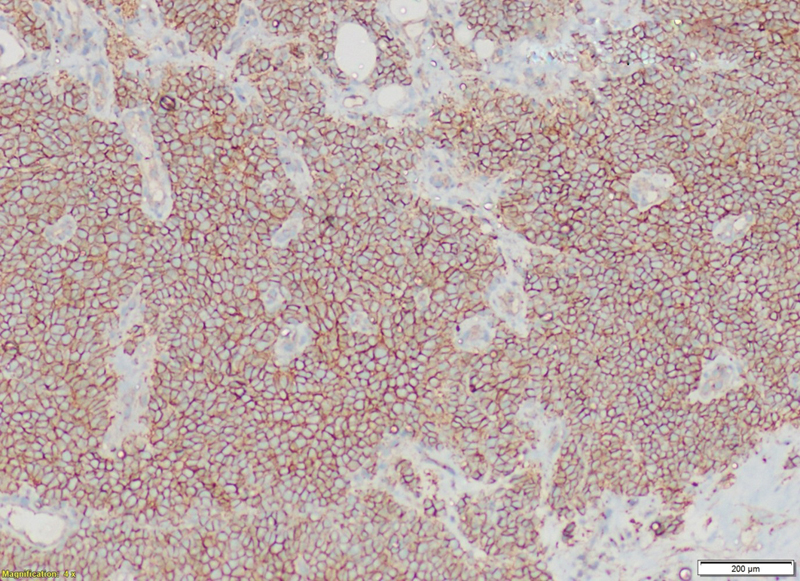
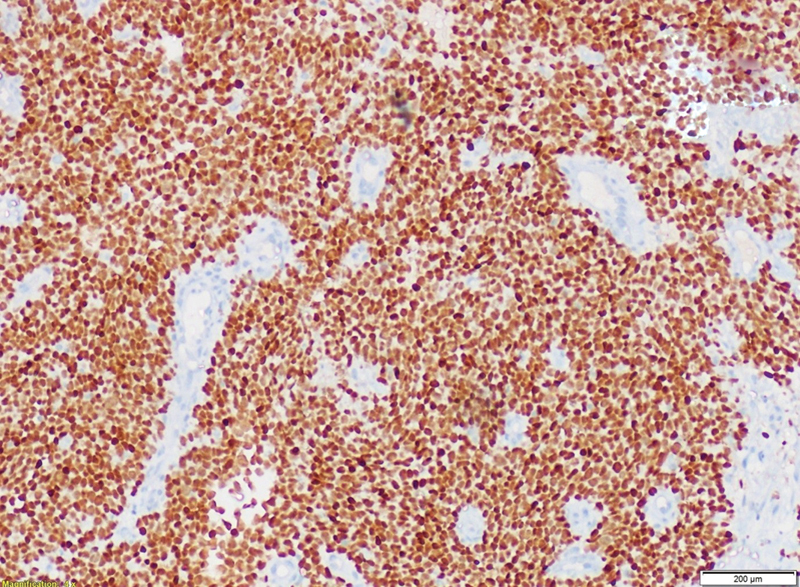
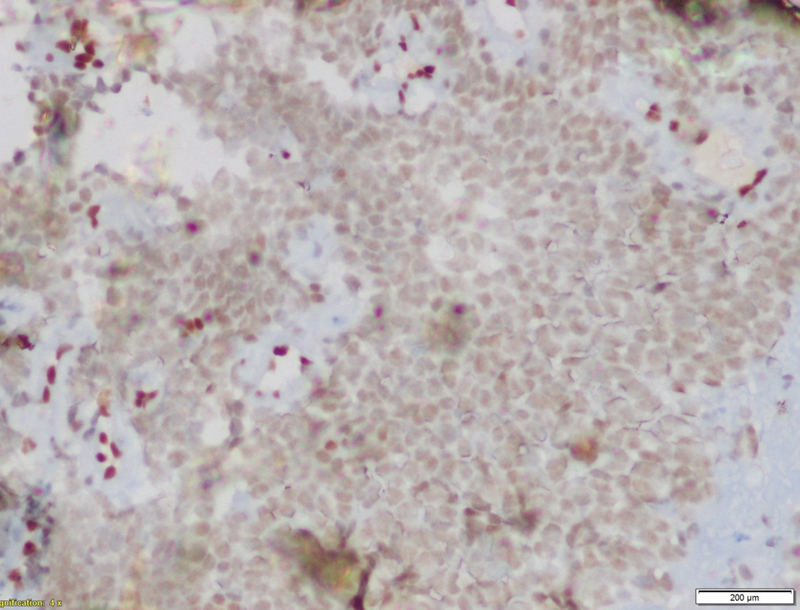
Tumor cells were immunonegative for transducer-like enhancer of split 1, epithelial membrane antigen (EMA), cytokeratin, and desmin. Tumor cells were immunopositive for CD99 ([Fig. 5]), NKX2.2 ([Fig. 6]), and faintly positive for FLI ([Fig. 7]), while negative for EMA ([Fig. 8]). Fluorescence in situ hybridization (FISH) revealed 72%-tumor cells showing EWSR1 gene or t(12q22) gene rearrangement ([Fig. 9]). CD99 and FLI-1 are sensitive however poorly specific IHC markers. In fact, combined NKX2.2 and CD99 positivity has highest specificity. The morphological and immunohistochemical profile was indicative of ES.
| Fig. 5Clinical photograph depicting swelling involving the third phalanx on dorsal surface of hand.
| Fig.6:Clinical photograph ventral surface of hand showing swelling involving third phalanx.
| Fig.7:Radiograph revealing bone destruction with sclerosis of proximal phalanx of middle finger and involvement of soft tissue component.
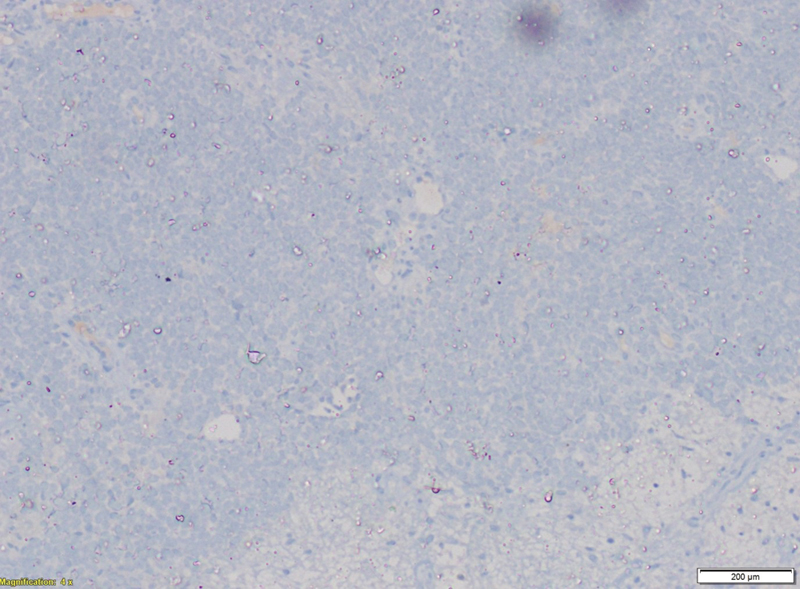
| Figure.8:Hematoxylin and eosin staining showing dense cellularity with small round blue cells uniformly arranged in sheets along with few areas of necrose.

| Figure.9:65-year-old male complaining of increased frequency of micturition, anorexia and weight loss due to small cell carcinoma of prostate. Sagittal T2-weighted showing enlarged prostate with urinary bladder invasion (black arrow) and extra-prostatic spread on right side with involvement of right peri-rectal fat (white arrow)
Contrast-enhanced tomography of chest and whole-body bone scan showed no evidence of metastatic disease. She was administered two cycles of neoadjuvant chemotherapy with inj. vincristine 1.5 mg/m2, inj. doxorubicin 75 mg/m2, inj. cyclophosphamide 1.2 g/m2 (day 1) alternating with inj. ifosfamide 1.8 g/m2, inj. etoposide100 mg/m2 (day1–day 5) every 3 weeks. As clinically there was no improvement, response assessment was done post two cycles that suggested stable disease. The patient is planned for amputation at right wrist joint followed by adjuvant chemotherapy with the same regimen.
Discussion
-
Question 1: What is the clinical differential diagnosis of such presentation in this age group?
-
Answer 1: Soft tissue or bone sarcoma including epithelioid sarcoma, melanoma, mucormycosis, and giant cell tumor of tendon sheath.
-
Question 2: What is the differential diagnosis of small round blue cell tumors?
-
Answer 2: Primitive neuroectodermal tumors, lymphoma, rhabdomyosarcoma, and synovial sarcoma.
-
Question 3: Which chromosomal abnormalities are seen in ES?
-
Answer 3: Distinct nonrandom chromosomal translocations involving the Ewing sarcoma gene (EWS) on chromosome number 22- t(11;22)(q24;q12) involving EWSR1-FLI1 in more than 80%-cases, while t(21;22)(q24;q12) involving EWSR1-ERG is seen in 10 to 15%-cases.
-
Alternate events include fusions of 22q12 with other erythroblast transformation-specific (ETS) gene partners—7p22, 17p22, 17p22,2q33 (ETV1, ETV4, FEV, PATZ1) in nearly 5% of cases.
-
Question 4: Which techniques can be used to diagnose chromosomal abnormalities in ES?
-
Answer 4: EWSR1 gene rearrangement by break apart FISH or reverse-transcription polymerase chain reaction.
-
ES is a part of the Ewing sarcoma family of tumors (EFT). Previously, it was considered primitive neuroectodermal cell in origin,[4] now believed to arise from mesenchymal stem cells.[5]
-
Question 5: What comprises ES family of tumors (EFT)?
-
Answer 5: It includes extraosseous ES, primitive neuroectodermal tumor, malignant small round tumor of thoracopulmonary region (Askin tumor) and atypical ES.
In 20% of patients, tumors are extraosseous and this occurs more frequently in adults.[6] [7]
Patients present with localized pain and swelling. A pathological fracture is reported in 10 to 15% of cases, and nonspecific constitutive symptoms, including fever, weight loss, night sweats, and fatigue, may be present. Blood tests may show elevated levels of alkaline phosphatase and serum lactate dehydrogenase.[8]
Common sites according to incidence of cases include bones of lower extremities (40–45%), pelvis (20–25%), chest wall (15–20%), and upper extremities (10%). Extra skeletal sites may include gastrointestinal tract, kidneys, and skeletal muscle. Metastasis to lungs, bones or bone marrow may be present in around 25% of cases. Nodal or liver involvement is rare.[9]
Hand and wrist involvement represents less than 1%-of all ES. Metacarpal and proximal phalanges of the thumb (28%) and middle finger (28%) are the most affected locations in the hand.[10] [11]
On radiographs, there are multiple, confluent, and lytic bone lesions collectively called “moth eaten appearance” are observed. Subperiosteal growth gives rise to the “Codman's triangle” and the “onion peel” that represent the displaced periosteum and resulting the proliferative reaction.
Induction chemotherapy is given for four cycles followed by local therapy at 12 weeks. Maintenance in the form of adjuvant chemotherapy with or without radiotherapy is recommended following surgery with the total duration of chemotherapy being 28 to 49 weeks. If the tumor is inoperable, local irradiation is given. Early studies suggested vincristine, doxorubicin, dactinomycin, and cyclophosphamide were active in treating ES.[12] [13] [14] The chemotherapy protocols have evolved over time as North American intergroup trial INT-OO91 established that adding IE (ifosfamide etoposide) to vincristine, doxorubicin, and cyclophosphamide (VDC) that improved 5-year event-free survival (EFS: 54 vs. 69%) for localized disease.[15] The current approach of interval compressed VDC/IE every 2 weeks was established by Children's Oncology Group AEWS0031 protocol that demonstrated better EFS of 73 versus 65%-for 2 weeks as opposed to 3 weeks.[16]
Literature review on ES reveals seven cases with finger involvement in adults. To the best of our knowledge, this is the eighth case. Baccari et al reported two cases of phalanx with a literature review of 15 cases in which only 6 were adults.[10] In a 22-year-old female with ES of index finger reported by Wolthuizen et al, in 2019, treatment was index-ray amputation followed by adjuvant chemotherapy.[17]
Conclusion
Preoperative chemotherapy is generally prescribed for the limb except for amputation surgery candidates. The response rates for primary tumor are minimal, for those who require amputation. Such patients should immediately undergo surgery.
No conflict of interest has been declared by the author(s).
Acknowledgment
Residents and faculty of Department of Medical Oncology.
Source(s) of support
None
Declarations
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity but anonymity cannot be guaranteed.
Competing Interests
None of the authors have any competing interests.
Authors' Contributions
SB and AC contributed to the conception of the study.SV, LAJ, LD, and SS were responsible for the acquisition. SA, LAJ, SBMC, LKN, AHR, and LKR drafted the work. LD, LAJ, and LKR substantively revised it. All authors have read and approved the manuscript.
References
- Campanacci M. Bone and soft tissue tumors. Springer-Verlag Wien.; 1999. DOI: 10.1007/978-3-7091-3846-5
- Mirra J. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia: Lea & Febiger; 1989
- Grünewald TGP, Cidre-Aranaz F, Surdez D. et al. Ewing sarcoma. Nat Rev Dis Primers 2018; 4 (01) 5
- Seroussi D, Renauld V, Hébrard W, Duport G, Cohrs D. [Ewing sarcoma of thumb: report of a case and review of the literature]. Ann Chir Plast Esthet 2004; 49 (04) 378-382 [Review. French]
- Lin PP, Wang Y, Lozano G. Mesenchymal stem cells and the origin of Ewing's Sarcoma. Sarcoma 2011; 2011: 276463
- Lynch AD, Gani F, Meyer CF, Morris CD, Ahuja N, Johnston FM. Extraskeletal versus skeletal Ewing sarcoma in the adult population: controversies in care. Surg Oncol 2018; 27 (03) 373-379
- Jahanseir K, Folpe AL, Graham RP. et al. Ewing sarcoma in older adults: a clinicopathologic study of 50 cases occurring in patients aged 340 years, with emphasis on histologic mimics. Int J Surg Pathol 2020; 28 (04) 352-360
- Biswas B, Shukla NK, Deo SVS. et al. Evaluation of outcome and prognostic factors in extraosseous Ewing sarcoma. Pediatr Blood Cancer 2014; 61 (11) 1925-1931
- Heidi V, Russell HV, Pappo AS, Nuchtern JG, Kornguth DG, Wang LL. Childhood cancers. Solid tumors of childhood. In: DeVita VT, Lawrence TS, Rosenberg SA. eds. Devita, Hellman & Rosenberg's Cancer: Principles & Practice of Oncology. 8th edition.. Philadelphia: Lippincott Williams & Wilkins; 2008
- Baccari S, Hamdi MF, Mabrouki Z, Daghfous M, Tarhouni L. Ewing's sarcoma of the finger: report of two cases and literature review. Orthop Traumatol Surg Res 2012; 98 (02) 233-237
- Fujii H, Honoki K, Kobata Y, Yajima H, Kido A, Takakura Y. Ewing sarcoma of the proximal phalanx: case report. J Plast Surg Hand Surg 2014; 48 (06) 441-443
- Chan RC, Sutow WW, Lindberg RD, Samuels ML, Murray JA, Johnston DA. Management and results of localized Ewing's sarcoma. Cancer 1979; 43 (03) 1001-1006
- Zucker JM, Henry-Amar M, Sarrazin D, Blache R, Patte C, Schweisguth O. Intensive systemic chemotherapy in localized Ewing's sarcoma in childhood. A historical trial. Cancer 1983; 52 (03) 415-423
- Rosen G, Caparros B, Mosende C, McCormick B, Huvos AG, Marcove RC. Curability of Ewing's sarcoma and considerations for future therapeutic trials. Cancer 1978; 41 (03) 888-899
- Grier HE, Krailo MD, Tarbell NJ. et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348 (08) 694-701
- Womer RB, West DC, Krailo MD. et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012; 30 (33) 4148-4154
- Wolthuizen R, Nieken J, Overbosch J, Pool SMW. An extraosseous Ewing sarcoma of the index finger masquerading as a benign tumor. J Hand Surg Am 2020 Apr;45:336.e1–366.e4.doi.org/10.1016/j.jhsa.2019.05.009.E pub 2019 Jul 18.PMID: 31327500
Address for correspondence
Publication History
Article published online:
14 March 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP,
India

| Fig. 1Clinical photograph depicting swelling involving the third phalanx on dorsal surface of hand.

| Fig.2:Clinical photograph ventral surface of hand showing swelling involving third phalanx.

| Fig.3:Radiograph revealing bone destruction with sclerosis of proximal phalanx of middle finger and involvement of soft tissue component.

| Figure.4:Hematoxylin and eosin staining showing dense cellularity with small round blue cells uniformly arranged in sheets along with few areas of necrose.

| Fig. 5Clinical photograph depicting swelling involving the third phalanx on dorsal surface of hand.

| Fig.6:Clinical photograph ventral surface of hand showing swelling involving third phalanx.

| Fig.7:Radiograph revealing bone destruction with sclerosis of proximal phalanx of middle finger and involvement of soft tissue component.

| Figure.8:Hematoxylin and eosin staining showing dense cellularity with small round blue cells uniformly arranged in sheets along with few areas of necrose.

| Figure.9:65-year-old male complaining of increased frequency of micturition, anorexia and weight loss due to small cell carcinoma of prostate. Sagittal T2-weighted showing enlarged prostate with urinary bladder invasion (black arrow) and extra-prostatic spread on right side with involvement of right peri-rectal fat (white arrow)
References
- Campanacci M. Bone and soft tissue tumors. Springer-Verlag Wien.; 1999. DOI: 10.1007/978-3-7091-3846-5
- Mirra J. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia: Lea & Febiger; 1989
- Grünewald TGP, Cidre-Aranaz F, Surdez D. et al. Ewing sarcoma. Nat Rev Dis Primers 2018; 4 (01) 5
- Seroussi D, Renauld V, Hébrard W, Duport G, Cohrs D. [Ewing sarcoma of thumb: report of a case and review of the literature]. Ann Chir Plast Esthet 2004; 49 (04) 378-382 [Review. French]
- Lin PP, Wang Y, Lozano G. Mesenchymal stem cells and the origin of Ewing's Sarcoma. Sarcoma 2011; 2011: 276463
- Lynch AD, Gani F, Meyer CF, Morris CD, Ahuja N, Johnston FM. Extraskeletal versus skeletal Ewing sarcoma in the adult population: controversies in care. Surg Oncol 2018; 27 (03) 373-379
- Jahanseir K, Folpe AL, Graham RP. et al. Ewing sarcoma in older adults: a clinicopathologic study of 50 cases occurring in patients aged 340 years, with emphasis on histologic mimics. Int J Surg Pathol 2020; 28 (04) 352-360
- Biswas B, Shukla NK, Deo SVS. et al. Evaluation of outcome and prognostic factors in extraosseous Ewing sarcoma. Pediatr Blood Cancer 2014; 61 (11) 1925-1931
- Heidi V, Russell HV, Pappo AS, Nuchtern JG, Kornguth DG, Wang LL. Childhood cancers. Solid tumors of childhood. In: DeVita VT, Lawrence TS, Rosenberg SA. eds. Devita, Hellman & Rosenberg's Cancer: Principles & Practice of Oncology. 8th edition.. Philadelphia: Lippincott Williams & Wilkins; 2008
- Baccari S, Hamdi MF, Mabrouki Z, Daghfous M, Tarhouni L. Ewing's sarcoma of the finger: report of two cases and literature review. Orthop Traumatol Surg Res 2012; 98 (02) 233-237
- Fujii H, Honoki K, Kobata Y, Yajima H, Kido A, Takakura Y. Ewing sarcoma of the proximal phalanx: case report. J Plast Surg Hand Surg 2014; 48 (06) 441-443
- Chan RC, Sutow WW, Lindberg RD, Samuels ML, Murray JA, Johnston DA. Management and results of localized Ewing's sarcoma. Cancer 1979; 43 (03) 1001-1006
- Zucker JM, Henry-Amar M, Sarrazin D, Blache R, Patte C, Schweisguth O. Intensive systemic chemotherapy in localized Ewing's sarcoma in childhood. A historical trial. Cancer 1983; 52 (03) 415-423
- Rosen G, Caparros B, Mosende C, McCormick B, Huvos AG, Marcove RC. Curability of Ewing's sarcoma and considerations for future therapeutic trials. Cancer 1978; 41 (03) 888-899
- Grier HE, Krailo MD, Tarbell NJ. et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348 (08) 694-701
- Womer RB, West DC, Krailo MD. et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012; 30 (33) 4148-4154
- Wolthuizen R, Nieken J, Overbosch J, Pool SMW. An extraosseous Ewing sarcoma of the index finger masquerading as a benign tumor. J Hand Surg Am 2020 Apr;45:336.e1–366.e4.doi.org/10.1016/j.jhsa.2019.05.009.E pub 2019 Jul 18.PMID: 31327500


 PDF
PDF  Views
Views  Share
Share

