Expression of phosphatase and tensin homolog, epidermal growth factor receptor, and Ki-67 in astrocytoma: A prospective study in a tertiary care hospital
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(02): 149-155
DOI: DOI: 10.4103/0971-5851.138983
Abstract
Background: Though the tumor grade is a key factor influencing the choice of therapies, particularly determining the use of adjuvant radiation and specific chemotherapy protocols, role of abnormality in phosphatase and tensin homolog (PTEN) expression and variation in epidermal growth factor receptor (EGFR) labeling index (LI) and Ki-67 LI in survival and clinical outcome have been studied by many researchers in the recent past. Aims: The aim was to evaluate the expression of PTEN, EGFR and Ki-67 in different grades of astrocytic tumors by means of immunohistochemistry and to judge their role in overall survival (OS).Materials and Methods: This study was conducted on 57 cases of different grades of astrocytomas. Expression of PTEN, EGFR and Ki-67 was assessed by immunohistochemistry on formalin fixed and paraffin-embedded sections and the OS was evaluated by Kaplan-Meier survival curves and log-rank test for 2.5 years from the date of primary resection. Results: Most of the tumors (59.6%; 34 cases out of 57) displayed WHO Grade IV features. Distribution of age, EGFR LI and Ki-67 LI expressed strong positive (≥0.5) correlation with the grade of tumors. However, the PTEN positivity was inversely related with the grade of the tumors. Lower WHO grades, lower values of Ki-67 LI, EGFR LI and PTEN positivity were associated with better survival. Conclusion: Expression of PTEN, EGFR LI and Ki-67 LI should be combined with the basic histopathological features including WHO grade to predict the prognosis and therapeutic outcome.
Keywords
Astrocytoma - epidermal growth factor receptor - glioblastoma - Ki-67 - phosphatase and tensin homologPublication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Though the tumor grade is a key factor influencing the choice of therapies, particularly determining the use of adjuvant radiation and specific chemotherapy protocols, role of abnormality in phosphatase and tensin homolog (PTEN) expression and variation in epidermal growth factor receptor (EGFR) labeling index (LI) and Ki-67 LI in survival and clinical outcome have been studied by many researchers in the recent past.
Aims:
The aim was to evaluate the expression of PTEN, EGFR and Ki-67 in different grades of astrocytic tumors by means of immunohistochemistry and to judge their role in overall survival (OS).
Materials and Methods:
This study was conducted on 57 cases of different grades of astrocytomas. Expression of PTEN, EGFR and Ki-67 was assessed by immunohistochemistry on formalin fixed and paraffin-embedded sections and the OS was evaluated by Kaplan–Meier survival curves and log-rank test for 2.5 years from the date of primary resection.
Results:
Most of the tumors (59.6%; 34 cases out of 57) displayed WHO Grade IV features. Distribution of age, EGFR LI and Ki-67 LI expressed strong positive (≥0.5) correlation with the grade of tumors. However, the PTEN positivity was inversely related with the grade of the tumors. Lower WHO grades, lower values of Ki-67 LI, EGFR LI and PTEN positivity were associated with better survival.
Conclusion:
Expression of PTEN, EGFR LI and Ki-67 LI should be combined with the basic histopathological features including WHO grade to predict the prognosis and therapeutic outcome.
INTRODUCTION
Gliomas and glioneuronal tumors constitute the largest and most heterogeneous group of primary central nervous system tumors[1] and diffuse astrocytic tumors (WHO Grades II-IV) comprise roughly 60% of all the primary intracranial tumors, with an annual incidence of 5-7/100,000 person-years.[2] Glioblastoma (GBM; Grade IV) is the most frequent neoplasm of astrocytic origin, comprising approximately 60-75% of the astrocytomas.[3] The two most powerful prognostic variables in astrocytic tumors are patient age and histologic grade. Age is inversely proportional to survival time.[2] Tumor grade is a key factor influencing the choice of therapies, particularly determining the use of adjuvant radiation and specific chemotherapy protocols.[3] Ki-67 is a useful marker for estimating proliferative index, which is roughly proportional to histologic grade.[4] In addition to histological grade and proliferating index the most frequent genetic alterations associated with the generation of GBM and progression of astrocytic tumors are deletions involving either large segments or an entire copy of chromosome 10, along with amplification of epidermal growth factor receptor (EGFR) gene on chromosome 7.[5] Particularly, loss of heterozygosity (LOH) around the mutated in multiple advanced cancers/phosphatase and tensin homolog (MMAC/PTEN), a novel tumor suppressor gene has been detected more frequently in patients with GBM than in patients with low-grade gliomas such as anaplastic astrocytoma (AA) and low-grade astrocytoma and the mutations on this gene were seen in high grade gliomas, but not in lower grade gliomas.[6,7] Overexpression of the proto-oncogene c-erbB1, encoding the EGFR, is proposed to be linked to the progression of lower grade astrocytomas to a higher grade. Hence, its expression has diagnostic, prognostic, and therapeutic implications.[8]
We have conducted this study to assess the expression of PTEN, EGFR and Ki-67 in different grades of astrocytic tumors by means of immunohistochemistry on formalin fixed and paraffin-embedded sections (FFPE) and to evaluate their prognostic role in astrocytomas.
MATERIALS AND METHODS
The present single-center study was carried out on 57 cases of astrocytoma after obtaining the proper approval from Ethical Committee of the institution and informed consent from the patients. All the clinically and radiologically suspected patients of brain tumors, who were admitted in the Department of neurosurgery for surgical intervention during the study period of 2.5 years (01.10.2009 to 31.03.2012) and subsequently diagnosed as astrocytoma of different grades on histopathological examination, were included in the study. Patients with a history of preoperative chemotherapy or radiotherapy were excluded from the study. In addition to relevant clinical features, detailed histopathological examination was carried on hematoxylin and eosin stained sections of 3-5 μm thickness prepared from FFPE tissue. Tumors were classified morphologically and graded according to the current WHO system.[9]
Immunohistochemical analysis
Immunohistochemical staining was done for Ki-67, EGFR and PTEN with prediluted ready-to-use monoclonal antibody (Bio-Genex, USA) on FFPE tissue sections mounted on poly-L-Lysine coated slides. Monoclonal antibodies employed were EPR3611 and EP38Y from rabbit and 28H6 from mouse for Ki-67, EGFR and PTEN respectively. Following the process of baking, dewaxing and rehydration, antigen retrieval was carried out by means of pressure-cooking. Incubation with monoclonal primary antibody in humidifying chamber was done for about 40-60 min at room temperature after using peroxide block. Secondary antibody conjugated with horse radish peroxidase (polymer-HRP) enzyme was applied for 30 min. Thereafter di-amino benzidine was added for 10 min as a substrate chromogen solution to produce a brown color reacting with the plenty of HRP molecules attached with the polymer. Finally the slides were immersed in Harris Hematoxylin for counterstaining. Result of Ki-67 and EGFR immunostaining was interpreted as labeling index (LI) (Ki-67 LI and EGFR LI) that was calculated as percentage of positively stained nuclei or cytoplasmic membranes respectively after counting at least 1000 cells in representative areas. PTEN immunostaining was reported as positive or negative depending on nuclear staining.
Follow-up for survival
After primary resection of the tumor mass, low-grade (Grades I and II) astrocytoma patients were treated with re-surgery in case of relapse. High grade (Grades III and IV) astrocytoma patients were managed with radiation therapy, with chemotherapy during or following radiation in addition to surgical resection. Only the overall survival (OS) was evaluated for 2.5 years from the date of primary resection to either date of death or last follow-up, whichever was earlier, but the disease free survival was not evaluated in the present study.
Statistical analysis
Statistical analysis was performed by using Kruskal-Wallis and Chi-square test using IBM SPSS statistics version 20 and MedCalc software. Correlations were judged by Spearman rank order correlation coefficient. Survival was assessed and compared using Kaplan–Meier curves and log-rank test. P < 0>
RESULTS
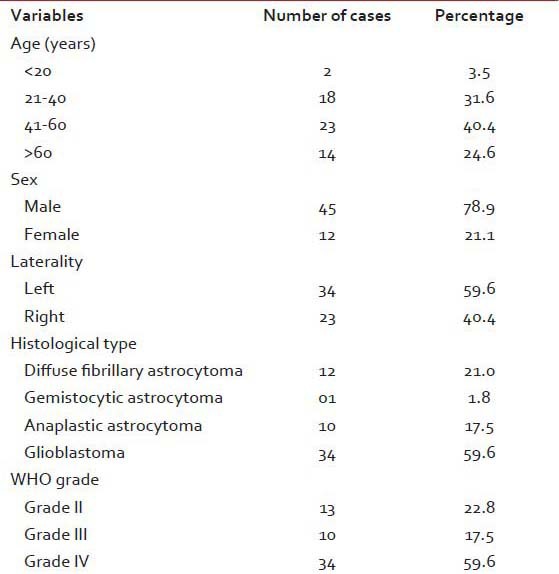
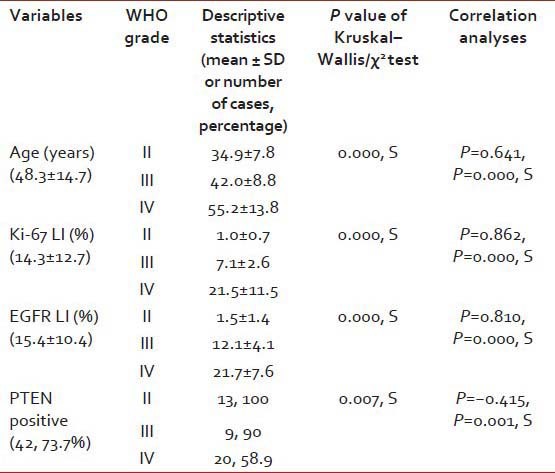
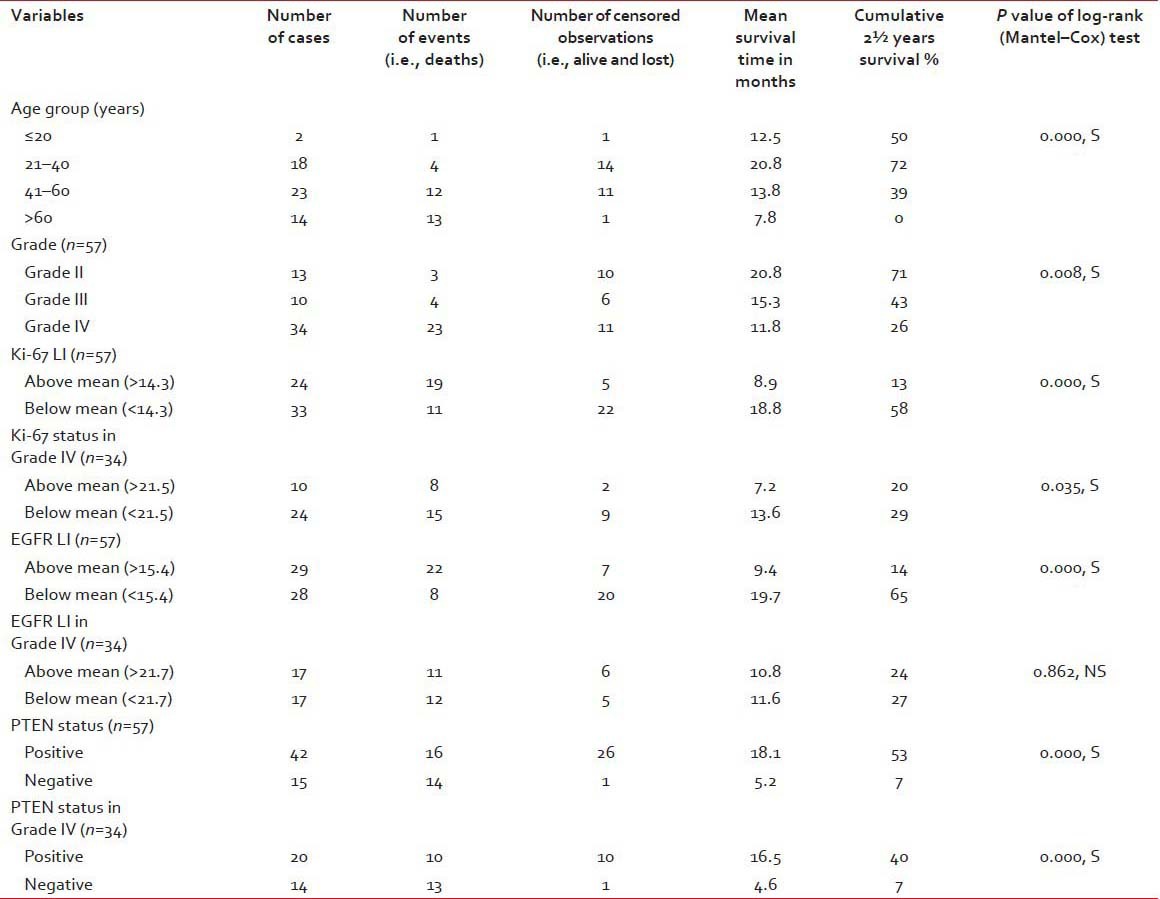
A total of 57 cases of astrocytoma were evaluated in the present study, of which 45 were males (78.9%) and the rest 12 were females (21.1%). Mean age of the study population was 48.3 years, and most of the cases (40.4%) were clustered in 41-60 years age group [Table 1]. A total of 34 (59.6%) lesions were localized in the left side, and 23 (40.4%) in the right. Most of the tumors (59.6%; 34 cases out of 57) displayed WHO Grade IV features followed by Grades II and III features, respectively. However, we had not received any Grade I astrocytoma in our study period. Distribution of age and expression of Ki-67, EGFR and PTEN in different grades of astrocytoma [Table 2 and Figure 1] revealed significant (<0 xss=removed>P value in Kruskal–Wallis/Chi-square test and in correlation analyses. Age, Ki-67 LI and EGFR LI expressed strong positive (≥0.5) correlation with the grade of tumors, that is, higher values of the three variables were associated with higher tumor grade and vice versa. However, the PTEN positivity was inversely related with the grade of the tumors. Kaplan–Meier plots [Table 3 and Figure 2] of OS were examined with log-rank tests of significance for patient age, grade of the tumors, Ki-67 LI, EGFR LI and PTEN expression. Cumulative 2½ years survival as well as mean survival time decreased with the increasing age groups, higher WHO grades, higher values of Ki-67 LI and EGFR LI. However, the PTEN positive cases were associated with better survival. Significant (<0 xss=removed>P values were observed between two groups in each set of Kaplan–Meier survival analysis derived by log-rank test except EGFR LI in Grade IV tumors between above mean and below mean groups.
Table 1
Clinical and histopathological variables of the astrocytoma patients
|
Table 2
Distribution of age and expression of Ki-67, EGFR and PTEN in different grades of tumors
|
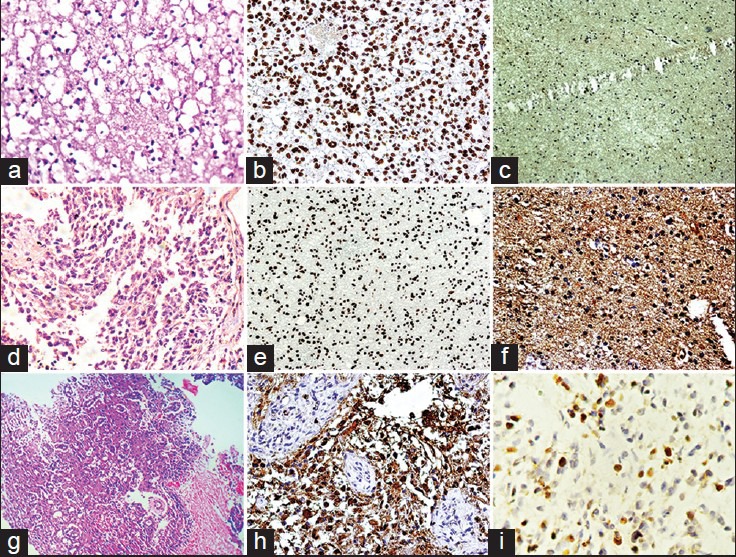
| Figure 1:(a) Photomicrograph of diffuse fibrillary astrocytoma (H and E, ×400) (b) phosphatase and tensin homolog (PTEN) expression in diffuse fibrillary astrocytoma (×400) (c) epidermal growth factor receptor (EGFR) immunostaining in a case of diffuse fibrillary astrocytoma (×100) (d) photomicrograph of anaplastic astrocytoma (H and E, ×100) (e) PTEN expression in anaplastic astrocytoma (×100) (f) EGFR immunostaining in a case of anaplastic astrocytoma (×400) (g) photomicrograph of glioblastoma (GBM) (H and E, ×100) (h) EGFR immunostaining in a case of GBM (×400) (i) Ki-67 expression in a case of glioblastoma (×400)
Table 3
Survival statistics of the patients derived from Kaplan–Meier survival analysis and log-rank test
|
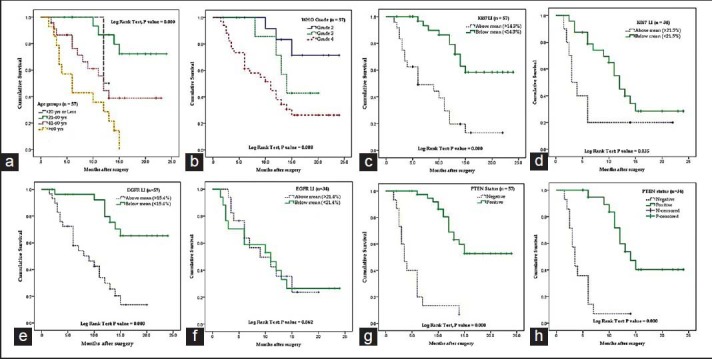
| Figure 2:Kaplan–Meier plots showing overall survival in relation to (a) age groups (b) WHO grade (c) Ki-67 labeling index (LI) in all cases (d) Ki-67 LI in Grade IV tumors (e) epidermal growth factor receptor (EGFR) LI in all cases (f) EGFR LI in Grade IV tumors (g) phosphatase and tensin homolog (PTEN) expression in all cases (h) PTEN expression in Grade IV tumors
DISCUSSION
The mean ages of patients with Grades I-IV were 12.96, 36.79, 41.52 and 49.55 years, respectively in the study by Ambroise et al.[10] Jalali et al.[11] observed the GBM cases within 13-69 years with a median age of 49.5 years and Srividya et al.[12] obtained the median age of 48 years in their study on newly diagnosed GBMs.
In a study on recurrent astrocytic tumors by Ralte et al.[13] revealed that the mean ages of Grades II-IV tumors were 31, 33.7 and 45.1 years respectively, and there was a striking male preponderance in all grades of astrocytic tumors. Lin et al.[6] noticed the median age of 52 years in cases of GBM and 39 years in AA. Male and female ratio of patients was 1.75:1.
In the present study, the mean ages of the Grades II-IV cases were 34.9, 42 and 55.2 years respectively, which are corroborating with the findings of the other relevant studies. It also displayed the striking male predominance. The occurrence of Grades II-IV tumors was 22.8%, 17.5% and 59.6%, respectively. This variation of incidence in our study might be explained by the small sample size and/or ethnic variation of the population in this region, which needs further long-term study.
Phosphatase and tensin homolog is a novel tumor suppressor gene located on 10q23 which is engaged in cell cycle arrest and apoptosis through negatively regulating the survival signaling in PI3K/AKT pathway in which it produces phosphatidylinositol 4,5-bisphosphate (PIP2) from phosphatidylinositol 3,4,5-triphosphate (PIP3) by the process of dephosphorylation. PIP2 maintains inactivity in the AKT pathway whereas PIP3 is a key regulator of the PI3K/AKT signaling pathway, which recruits AKT (also called protein kinase B) to the membrane surface. Activated AKT regulates several downstream pathways controlling the cell cycle progression, protein synthesis, cell survival and apoptosis. Loss of PTEN expression, through deletion, mutation or methylation essentially mimics activation of the AKT pathway as a result of the accumulation of PIP3, while retention of PTEN maintains AKT inactivity. The active AKT leads to tumorigenesis due to loss of proliferative and apoptotic controls of cell growth especially in breast, ovaries, prostate, pancreas, endometrium, skin, and most notably, brain where inactivation of the PTEN is one of the molecular hallmarks of GBM. PTEN-dependent abnormalities of signaling are very frequent in GBM, with mutation occurring in between 5% and 40% of all GBM cases, and LOH in 60-80% of all cases.[3,12,14] Evaluation of cellular PTEN levels through expression profiling platform as well by immunohistochemistry have been found to be of prognostic significance in several other studies.[14] Srividya et al.[12] observed that the homozygous PTEN deletion is a highly significant prognostic marker for poor clinical outcome in newly diagnosed cases of GBM. Further, multivariate analysis revealed the independent prognostic value of 10q23/PTEN homozygous deletion with significant P = 0.033.
Lin et al.[6] observed a significantly (P < 0 xss=removed>et al.[7] demonstrated the overall incidence of PTEN mutations in 31% (13 of 42) of adult GBM and in 23% (3 of 13) of adult AA cases. No mutations were found in the 21 cases of low-grade adult gliomas (astrocytomas and oligodendrogliomas) and in the 22 cases of childhood gliomas. PTEN alteration was significantly less frequent in AAs (18%) than in the GBMs (34%) and similarly EGFR gene amplification was significantly less common in the AAs (17%) than in the GBMs (41%) in a well-known study by Smith et al.[15] PTEN mutation, EGFR amplification and age of the patients came out as predictors of survival in their study on AA and GBM patients.
Though the previous literature had advocated the independent prognostic role of PTEN loss in GBM, other studies have demonstrated the importance of EGFR amplification as well as other methods of AKT pathway activation in tumorigenesis. A very recent study even found a significant correlation between PTEN loss and EGFR amplification.[14]
Agosti et al.[16] observed positive EGFR expression in 37%-cases of GBMs whereas Grades I-III astrocytic tumors were EGFR negative on immunohistochemical analysis using a monoclonal antibody. Similar study conducted by Torp et al.[8] revealed EGFR positivity in 75%-cases of AAs and 63%-cases of GBMs. Bouvier-Labit et al.[17] found anti-EGFR immunoreactivity in 35%-cases of GBMs. In a study on 157 gliomas, proliferating cell nuclear antigen (PCNA) and/or EGFR were expressed in a minority of low-grade astrocytomas, while AA and GBM displayed an intense immunoreactivity for the two antigens in >85%-of cases and their expression significantly correlated with morphological grading.[18] Similarly, Maiti et al.[19] noticed higher levels of EGFR expression and PCNA labeling index with increasing grades of astrocytomas with a significantly high percentage of cells showing positive staining for both EGFR and PCNA in GBM and Grade III astrocytomas compared to Grade II astrocytomas.
In our study, PTEN positivity was observed in 100%, 90%-and 58.9%-of the Grades II-IV tumors respectively which were statistically significant. On correlation analysis there was an inverse relationship between PTEN expression and grade of the tumors. PTEN positive cases were also associated with significantly better survival in all the astrocytic tumors as well as in Grade IV tumors. Mean EGFR LI was 1.5, 12.1 and 21.7 in Grades II-IV tumors in the present study showing significant P value in Kruskal–Wallis test. EGFR LI expressed strong positive (≥0.5) correlation with the grade of tumors and higher values of EGFR LI were significantly associated with the poorer survival. These findings are quite similar with that of the previous studies although very few immunohistochemical studies had been conducted for PTEN.
A total of 16 reviewed articles comprising a total of 915 patients revealed that the increase in MIB-1 LI was directly related with the increasing grade of malignancy. Most studies also demonstrated positive correlations between MIB-1 LI and survival.[20] The MIB-1 antibody recognizes the Ki-67 antigen in FFPE tissue. In an Indian study, mean values of MIB-1 LI were 3.8, 2.8, 7.5, and 13.9 in Grades I-IV astrocytomas, respectively. The study emphasized that MIB-1 LI was not dependent on factors such as age and sex and was solely dependent on histological grade.[10] In a study on 271 patients, the mean MIB-1 LI of diffuse astrocytomas of Grades II-IV were 2.3%, 6%, and 9.1%, respectively. There was a statistically significant difference in the distribution of MIB-1 LI between different grades of tumors and subsequently the survival was also significantly related with the MIB-1 LI.[4] Ralte et al.[13] observed a statistically significant inverse correlation of MIB-1 LI with interval to recurrence in a study of 64 cases of recurrent astrocytic tumors. The MIB-1 LI of initial tumors displayed a positive correlation with histologic grade. In a study of Ki-67 (MIB-1) and p53 labeling indexes in the stereotactic biopsy specimens, Tihan et al.[21] have demonstrated that the tumors with low Ki-67 LI (<15 xss=removed>P < 0 xss=removed>et al.[22] Rathi et al.[23] noticed the mean MIB-1 LI values of 1.75%, 8.74% and 20.54%-in astrocytomas, AAs and GBMs respectively. The statistical analysis also revealed that the mean MIB-1 LI increased with histological grade of malignancy.
The present study revealed 1.0, 7.1 and 21.5%-of Ki-67 LI in Grades II-IV tumors respectively and Ki-67 LI also displayed strong positive (≥0.5) correlation with the grade of tumors. Kaplan–Meier survival analysis with log-rank test demonstrated that higher values of Ki-67 LI were associated with the significantly poorer survival in all the cases as well as in Grade IV tumors. The findings of the contemporary studies are comparable with that of the present study.
CONCLUSION
Mainly, the five parameters namely age, WHO grade, PTEN expression, EGFR LI and Ki-67 LI have been evaluated in the present study, which revealed that patient survival was definitely related with all the above parameters. Thus, it can be safely concluded that expression of PTEN, EGFR and Ki-67 LI should be combined with the basic histopathological features including WHO grade to predict the prognosis and therapeutic outcome. A multicentric prospective and long-term follow-up study with larger numbers of patients is required to get the precise results and for validation of the conclusions reached in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Haberland C. Tumors of the central nervous system. In: Haberland C, editor. Clinical Neuropathology: Text and Color Atlas. New York, NY: Demos Medical Publishing; 2007. p. 213-59.
- Brat DJ, Perry A. Astrocytic and oligodendroglial tumors. In: Perry A, Brat DJ, editors. Practical Surgical Neuropathology: A Diagnostic Approach. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. p. 63-102.
- Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, Bigner DD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4 th ed. Lyon, France: International Agency for Research on Cancer; 2007. p. 33-49.
- Giannini C, Scheithauer BW, Burger PC, Christensen MR, Wollan PC, Sebo TJ, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol 1999;58:46-53.
- Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, et al. Differential expression of MMAC/PTEN in glioblastoma multiforme : r0 elationship to localization and prognosis. Cancer Res 1999;59:1820-4.
- Lin H, Bondy ML, Langford LA, Hess KR, Delclos GL, Wu X, et al. Allelic deletion analyses of MMAC/PTEN and DMBT1 loci in gliomas : r0 elationship to prognostic significance. Clin Cancer Res 1998;4:2447-54.
- Rasheed BK, Stenzel TT, McLendon RE, Parsons R, Friedman AH, Friedman HS, et al. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res 1997;57:4187-90.
- Torp SH, Bringedal K, Dalen A. Immunohistochemical detection of epidermal growth factor receptor in human high-grade astrocytomas: A comparison between frozen- and paraffin sections. J Exp Clin Cancer Res 2005;24:89-92.
- Kleihues P, Louis DN, Wiestler OD, Burger PC, Scheithauer BW. WHO grading of tumours of the central nervous system. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4 th ed. Lyon, France : I0 nternational Agency for Research on Cancer; 2007. p. 10-1.
- ;Ambroise MM, Khosla C, Ghosh M, Mallikarjuna VS, Annapurneswari S. Practical value of MIB-1 index in predicting behavior of astrocytomas. Indian J Pathol Microbiol 2011;54:520-5.
- Jalali R, Basu A, Gupta T, Munshi A, Menon H, Sarin R, et al. Encouraging experience of concomitant Temozolomide with radiotherapy followed by adjuvant Temozolomide in newly diagnosed glioblastoma multiforme : s0 ingle institution experience. Br J Neurosurg 2007;21:583-7.
- Srividya MR, Thota B, Shailaja BC, Arivazhagan A, Thennarasu K, Chandramouli BA, et al. Homozygous 10q23/PTEN deletion and its impact on outcome in glioblastoma : a0 prospective translational study on a uniformly treated cohort of adult patients. Neuropathology 2011;31:376-83.
- Ralte AM, Sharma MC, Karak AK, Mehta VS, Sarkar C. Clinicopathological features, MIB-1 labeling index and apoptotic index in recurrent astrocytic tumors. Pathol Oncol Res 2001;7:267-78.
- Carico C, Nuño M, Mukherjee D, Elramsisy A, Dantis J, Hu J, et al. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS One 2012;7:e33684.
- Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 2001;93:1246-56.
- Agosti RM, Leuthold M, Gullick WJ, Yasargil MG, Wiestler OD. Expression of the epidermal growth factor receptor in astrocytic tumours is specifically associated with glioblastoma multiforme. Virchows Arch A Pathol Anat Histopathol 1992;420:321-5.
- Bouvier-Labit C, Chinot O, Ochi C, Gambarelli D, Dufour H, Figarella-Branger D. Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol 1998;24:381-8.
- Mottolese M, Natali PG, Coli A, Bigotti G, Benevolo M, Cione A, et al. Comparative analysis of proliferating cell nuclear antigen and epidermal growth factor receptor expression in glial tumours : c0 orrelation with histological grading. Anticancer Res 1998;18:1951-6.
- Maiti AK, Ghosh K, Chatterjee U, Chakrobarti S, Chatterjee S, Basu S. Epidermal growth factor receptor and proliferating cell nuclear antigen in astrocytomas. Neurol India 2008;56:456-62.
- Johannessen AL, Torp SH. The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 2006;12:143-7.
- Tihan T, Davis R, Elowitz E, DiCostanzo D, Moll U. Practical value of Ki-67 and p53 labeling indexes in stereotactic biopsies of diffuse and pilocytic astrocytomas. Arch Pathol Lab Med 2000;124:108-13.
- Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme : a0 Ki-67 study. Neuropathol Appl Neurobiol 1990;16:123-33.
- Rathi KR, Radotra BD, Khosla VK. Proliferative index in astrocytic tumours. Indian J Pathol Microbiol 2007;50:754-8.

| Figure 1:(a) Photomicrograph of diffuse fibrillary astrocytoma (H and E, ×400) (b) phosphatase and tensin homolog (PTEN) expression in diffuse fibrillary astrocytoma (×400) (c) epidermal growth factor receptor (EGFR) immunostaining in a case of diffuse fibrillary astrocytoma (×100) (d) photomicrograph of anaplastic astrocytoma (H and E, ×100) (e) PTEN expression in anaplastic astrocytoma (×100) (f) EGFR immunostaining in a case of anaplastic astrocytoma (×400) (g) photomicrograph of glioblastoma (GBM) (H and E, ×100) (h) EGFR immunostaining in a case of GBM (×400) (i) Ki-67 expression in a case of glioblastoma (×400)

| Figure 2:Kaplan–Meier plots showing overall survival in relation to (a) age groups (b) WHO grade (c) Ki-67 labeling index (LI) in all cases (d) Ki-67 LI in Grade IV tumors (e) epidermal growth factor receptor (EGFR) LI in all cases (f) EGFR LI in Grade IV tumors (g) phosphatase and tensin homolog (PTEN) expression in all cases (h) PTEN expression in Grade IV tumors
References
- Haberland C. Tumors of the central nervous system. In: Haberland C, editor. Clinical Neuropathology: Text and Color Atlas. New York, NY: Demos Medical Publishing; 2007. p. 213-59.
- Brat DJ, Perry A. Astrocytic and oligodendroglial tumors. In: Perry A, Brat DJ, editors. Practical Surgical Neuropathology: A Diagnostic Approach. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. p. 63-102.
- Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, Bigner DD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4 th ed. Lyon, France: International Agency for Research on Cancer; 2007. p. 33-49.
- Giannini C, Scheithauer BW, Burger PC, Christensen MR, Wollan PC, Sebo TJ, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol 1999;58:46-53.
- Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, et al. Differential expression of MMAC/PTEN in glioblastoma multiforme : r0 elationship to localization and prognosis. Cancer Res 1999;59:1820-4.
- Lin H, Bondy ML, Langford LA, Hess KR, Delclos GL, Wu X, et al. Allelic deletion analyses of MMAC/PTEN and DMBT1 loci in gliomas : r0 elationship to prognostic significance. Clin Cancer Res 1998;4:2447-54.
- Rasheed BK, Stenzel TT, McLendon RE, Parsons R, Friedman AH, Friedman HS, et al. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res 1997;57:4187-90.
- Torp SH, Bringedal K, Dalen A. Immunohistochemical detection of epidermal growth factor receptor in human high-grade astrocytomas: A comparison between frozen- and paraffin sections. J Exp Clin Cancer Res 2005;24:89-92.
- Kleihues P, Louis DN, Wiestler OD, Burger PC, Scheithauer BW. WHO grading of tumours of the central nervous system. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4 th ed. Lyon, France : I0 nternational Agency for Research on Cancer; 2007. p. 10-1.
- ;Ambroise MM, Khosla C, Ghosh M, Mallikarjuna VS, Annapurneswari S. Practical value of MIB-1 index in predicting behavior of astrocytomas. Indian J Pathol Microbiol 2011;54:520-5.
- Jalali R, Basu A, Gupta T, Munshi A, Menon H, Sarin R, et al. Encouraging experience of concomitant Temozolomide with radiotherapy followed by adjuvant Temozolomide in newly diagnosed glioblastoma multiforme : s0 ingle institution experience. Br J Neurosurg 2007;21:583-7.
- Srividya MR, Thota B, Shailaja BC, Arivazhagan A, Thennarasu K, Chandramouli BA, et al. Homozygous 10q23/PTEN deletion and its impact on outcome in glioblastoma : a0 prospective translational study on a uniformly treated cohort of adult patients. Neuropathology 2011;31:376-83.
- Ralte AM, Sharma MC, Karak AK, Mehta VS, Sarkar C. Clinicopathological features, MIB-1 labeling index and apoptotic index in recurrent astrocytic tumors. Pathol Oncol Res 2001;7:267-78.
- Carico C, Nuño M, Mukherjee D, Elramsisy A, Dantis J, Hu J, et al. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS One 2012;7:e33684.
- Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 2001;93:1246-56.
- Agosti RM, Leuthold M, Gullick WJ, Yasargil MG, Wiestler OD. Expression of the epidermal growth factor receptor in astrocytic tumours is specifically associated with glioblastoma multiforme. Virchows Arch A Pathol Anat Histopathol 1992;420:321-5.
- Bouvier-Labit C, Chinot O, Ochi C, Gambarelli D, Dufour H, Figarella-Branger D. Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol 1998;24:381-8.
- Mottolese M, Natali PG, Coli A, Bigotti G, Benevolo M, Cione A, et al. Comparative analysis of proliferating cell nuclear antigen and epidermal growth factor receptor expression in glial tumours : c0 orrelation with histological grading. Anticancer Res 1998;18:1951-6.
- Maiti AK, Ghosh K, Chatterjee U, Chakrobarti S, Chatterjee S, Basu S. Epidermal growth factor receptor and proliferating cell nuclear antigen in astrocytomas. Neurol India 2008;56:456-62.
- Johannessen AL, Torp SH. The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 2006;12:143-7.
- Tihan T, Davis R, Elowitz E, DiCostanzo D, Moll U. Practical value of Ki-67 and p53 labeling indexes in stereotactic biopsies of diffuse and pilocytic astrocytomas. Arch Pathol Lab Med 2000;124:108-13.
- Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme : a0 Ki-67 study. Neuropathol Appl Neurobiol 1990;16:123-33.
- Rathi KR, Radotra BD, Khosla VK. Proliferative index in astrocytic tumours. Indian J Pathol Microbiol 2007;50:754-8.


 PDF
PDF  Views
Views  Share
Share

