Fluorodeoxyglucose-positron emission tomography in carcinoma nasopharynx: Can we predict outcomes and tailor therapy based on postradiotherapy fluorodeoxyglucose-positron emission tomography?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 47-52
DOI: DOI: 10.4103/0971-5851.177030
Abstract
Background: Positron emission tomography-computed tomography (PET-CT) is an emerging modality for staging and response evaluation in carcinoma nasopharynx. This study was conducted to evaluate the impact of PET-CT in assessing response and outcomes in carcinoma nasopharynx. Materials and Methods: Forty-five patients of nonmetastatic carcinoma nasopharynx who underwent PET-CT for response evaluation at 10-12 weeks posttherapy between 2004 and 2009 were evaluated. Patients were classified as responders (Group A) if there was a complete response on PET-CT or as nonresponders (Group B) if there was any uptake above the background activity. Data regarding demographics, treatment, and outcomes were collected from their records and compared across the Groups A and B. Results: The median age was 41 years. 42 out of 45 (93.3%) patients had WHO Grade 2B disease (undifferentiated squamous carcinoma). 24.4%, 31.1%, 15.6, and 28.8% patients were in American Joint Committee on Cancer Stage IIb, III, Iva, and IVb. All patients were treated with neoadjuvant chemotherapy followed by concomitant chemoradiotherapy. Forty-five patients, 28 (62.2%) were classified as responders, whereas 17 (37.8%) were classified as nonresponders. There was no significant difference in the age, sex, WHO grade, and stage distribution between the groups. Compliance to treatment was comparable across both groups. The median follow-up was 25.3 months (759 days). The disease-free survival (DFS) of the group was 57.3% at 3 years. The DFS at 3 years was 87.3% and 19.7% for Group A and B, respectively (log-rank test, P < 0.001). Univariate and multivariate analysis revealed Groups to be the only significant factor predicting DFS (P value 0.002 and < 0.001, respectively). In Group B, the most common site of disease failure was distant (9, 53%). Conclusion: PET-CT can be used to evaluate response and as a tool to identify patients at higher risk of distant failure. Further, this could be exploited to identify patients who may need treatment intensification. This needs to be validated prospectively.
Keywords
Carcinoma nasopharynx - positron emission tomography-computed tomography - predictive value - response evaluationPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Positron emission tomography-computed tomography (PET-CT) is an emerging modality for staging and response evaluation in carcinoma nasopharynx. This study was conducted to evaluate the impact of PET-CT in assessing response and outcomes in carcinoma nasopharynx.
Materials and Methods:
Forty-five patients of nonmetastatic carcinoma nasopharynx who underwent PET-CT for response evaluation at 10-12 weeks posttherapy between 2004 and 2009 were evaluated. Patients were classified as responders (Group A) if there was a complete response on PET-CT or as nonresponders (Group B) if there was any uptake above the background activity. Data regarding demographics, treatment, and outcomes were collected from their records and compared across the Groups A and B.
Results:
The median age was 41 years. 42 out of 45 (93.3%) patients had WHO Grade 2B disease (undifferentiated squamous carcinoma). 24.4%, 31.1%, 15.6, and 28.8% patients were in American Joint Committee on Cancer Stage IIb, III, Iva, and IVb. All patients were treated with neoadjuvant chemotherapy followed by concomitant chemoradiotherapy. Forty-five patients, 28 (62.2%) were classified as responders, whereas 17 (37.8%) were classified as nonresponders. There was no significant difference in the age, sex, WHO grade, and stage distribution between the groups. Compliance to treatment was comparable across both groups. The median follow-up was 25.3 months (759 days). The disease-free survival (DFS) of the group was 57.3% at 3 years. The DFS at 3 years was 87.3% and 19.7% for Group A and B, respectively (log-rank test, P < 0.001). Univariate and multivariate analysis revealed Groups to be the only significant factor predicting DFS (P value 0.002 and < 0.001, respectively). In Group B, the most common site of disease failure was distant (9, 53%).
Conclusion:
PET-CT can be used to evaluate response and as a tool to identify patients at higher risk of distant failure. Further, this could be exploited to identify patients who may need treatment intensification. This needs to be validated prospectively.
INTRODUCTION
Carcinoma nasopharynx is a rare cancer with a worldwide incidence of < 1 per 100,000. The incidence peaks in Southern China with the undifferentiated type (WHO Type 2.2) being the most common.[1] In India, the incidence is low except for the North-Eastern provinces.[2]
Despite improvement in local control rates with intensification in the form of chemotherapy (adjuvant or neoadjuvant) or radiotherapy (dose escalation and altered fractionation), the local and distant failure rates continue to be high. Thus, a definite distinction is required between patients with persistent and recurrent disease at follow-up, as the prognostic and therapeutic implications may be different for these groups of patients. Magnetic resonance imaging (MRI) and computed tomography (CT) scans are the conventional imaging procedures used for follow-up. However, positron emission tomography-CT (PET-CT) has been found to be better than these for detection of residual and recurrent disease with a sensitivity and specificity of 100% and 93.4%.[3]
The problem with PET-CT are the false positive results due to infections and tissue edema that reduce its specificity.[4] Another problem is regarding the timing of the scan. After radiation therapy, there seems to be a period when there is reduced uptake of fluorodeoxyglucose (FDG) even in the viable cells due to the stunning effect due to vascular damage and effect on cellular glucose transport by the radiation.[5] Due to this, there may be low-grade uptake which is more often than not interpreted as normal even though it may represent disease. Biopsies may prove inconclusive in such situations as they may miss the focus of active tumor and lead to erroneous decisions.
We conducted a retrospective analysis of patients of carcinoma nasopharynx who had received complete treatment at our institution to assess the prognostic and predictive value of this low-grade uptake visualized on PET-CT.
MATERIALS AND METHODS
Forty-five patients with diagnosed carcinoma nasopharynx (American Joint Committee on Cancer [AJCC] Stage IIb-Ivb) who were treated between April 2004 and December 2009 with neoadjuvant chemotherapy followed by concomitant chemoradiotherapy followed by evaluation with PET-CT after 10-12 weeks were evaluated for this retrospective analysis. Data were recorded with respect to diagnosis, staging, treatment, and follow-up from the case records obtained from the medical records department at Tata Memorial Centre.
Diagnosis and staging
All patients were diagnosed by a biopsy either from primary or nodes. Subsequently, they underwent staging with CT scans of head and neck, chest X-ray, and bone scan. MRI was done for patients to rule out intracranial extension. Staging PET-CT was done for patients whenever feasible. Subsequently, patients were staged according to the AJCC sixth edition which was in vogue during the time of this study.
Treatment
All patients received neoadjuvant chemotherapy with paclitaxel, ifosfamide, and cisplatinum for 2 cycles every 3 weeks.[6] Adequate supportive care was used with antiemetics, G-cerebrospinal fluid, and antibiotics whenever clinically warranted.
Radiotherapy was administered 2 weeks after completion of neoadjuvant chemotherapy to a dose equivalent of 66-70 Gy/33-35 number over 6-7 weeks with conventional, three-dimensional (3D) conformal radiotherapy or intensity modulated radiotherapy.
Along with radiotherapy concomitant chemotherapy was administered with weekly cisplatinum (30 mg/m2).
Follow-up
All patients included in this analysis underwent a follow-up PET-CT after 10-12 weeks of completion of radiotherapy. Subsequently, PET-CT was repeated annually or when the patient presented with signs and symptoms attributable to recurrence.
Positron emission tomography-computed tomography protocol and interpretation
Preparation of the patient was done according to the Society of Nuclear Medicine guidelines[7] with a patient fasting for 6-8 h and diabetic patients having a blood glucose levels < 160 mg/ml. The patients were injected intravenously with 18F-FDG, dose of 5 MBq/kg body weight. Scans were obtained 60-90 min postinjection in a 3D mode on a dedicated PET/CT scanner (Discovery ST, GE, Milwaukee, USA). A dedicated CT scan of the peripheral nervous system (PNS) and neck was obtained simultaneously during the staging study. A breath-hold CT of the chest was also taken. Two set of studies were done for the patient, at staging and after completion of therapy. Both the studies were obtained using the same acquisition and interpretation protocols. The studies were reconstructed using ordered-subsets expectation maximization and provided maximum intensity projection images and reconstruction in all three planes — axial, sagittal, and coronal sections.
The study was interpreted by a nuclear medicine physician and radiologist conjointly. Qualitative assessment of the study was done by identifying areas of focal increased tracer localization — at the primary site and nodal site or metastatic lesions if any on the staging PET/CT scan. The morphological details of the primary mass and other FDG avid sites were noted from the correlative CT images of the PET/CT study. Patients identified with distant metastases were not included in this study.
The study was evaluated quantitatively using the standard uptake value (SUV), a ratio of the amount of uptake in the region of interest (ROI) to the total injected dose and corrected for body weight. The volume based SUV's were calculated automatically by placing an ROI over the primary site and metastatic nodal sites if any.
The posttreatment scans were interpreted in comparison to the staging scan. The patterns of FDG concentration were noted — no uptake suggesting the absence of disease and focal uptake depicting residual disease. Diffuse uptake at the tumor sites was considered to be due to postradiation inflammatory changes. Anatomical changes at the primary and nodal sites were also noted simultaneously. Patients were thus grouped into Responders — no uptake at the primary and nodal sites; and Nonresponders — those who showed residual tracer concentration at either the primary or nodal sites. Any other areas of FDG uptake seen in the body suggested distant metastases and were suggestive of disease progression.
Statistics
SPSS version 14 (IBM) was used to analyze the data. Chi-square test was used to check if the demographic variables (age, sex, and stage) were equally distributed between the two groups. Kaplan-Meier plots were used to calculate the disease-free survival (DFS) and overall survival (OS). Survival times for DFS and OS were calculated from the initiation of chemotherapy. Univariate and multivariate analyses were performed using the same software.
RESULTS
Between April 2004 and December 2009, 45 patients were identified from the medical records Department of Tata Memorial Hospital, who had undergone PET-CT for response evaluation as per the above-stated protocol. This group was divided into responders (Group A) and nonresponders (Group B) as stated above. Twenty-eight (62.2%) patients were classified as Group A while 17 (37.8%) patients were classified as Group B. Both these groups were followed up until September 2010 when this analysis was performed.
Demographics
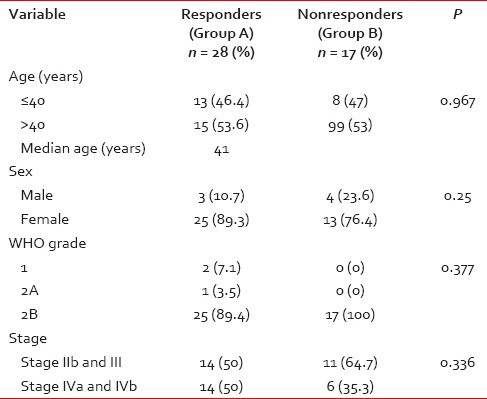
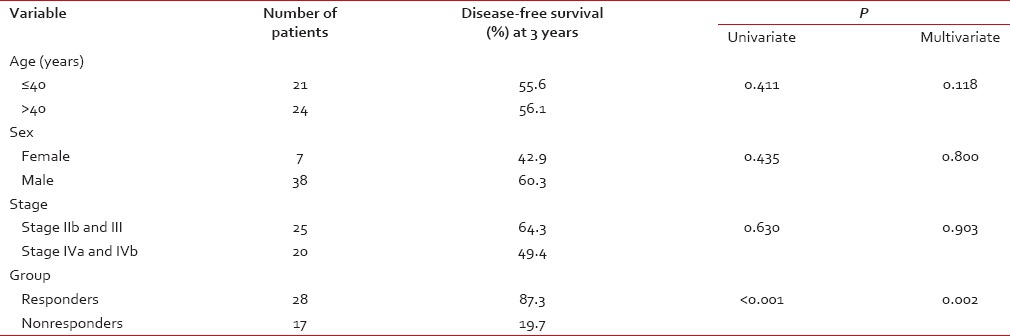
The median age of the study population was 41 years (range: 19-70 years). All patients had biopsy-proven carcinoma. WHO Grade 2B was the most common histology (93.3%). The patients were classified with AJCC staging as having Stage IIb (24.4.%), III (31.1%), IVa (15.6%), and IVb (28.8%). Essentially, this represented a group of patients with locally advanced nasopharyngeal carcinoma. There was no significant difference in stage across the two groups [Table 1]. Nine (20%) underwent staging PET-CT as routine staging PET-CT was not in vogue at our center at the time of this study.
Table 1
Patient characteristics compared between Group A and Group B
The median follow-up of the whole group was 25.3 months (759 days).
At the posttreatment evaluation with PET-CT the mean SUVmax in Group B was 1.4 at the primary site and 2.1 at nodal stations. There was no residual uptake in Group A.
Survival
The DFS of the whole group was 57.3% (median 14.5 months) at 3 years [Figure 1]. The DFS of the two groups was 87.3% (Group A) and 19.7% (Group B) at 3 years, respectively [Figure 2]. The difference in DFS between the two response groups was significant (log-rank test < 0.001) with the DFS being better in Group A. The OS of the whole group was 76.6% (median 25.3 months) at 3 years. The OS of the two groups was 86.3% (Group A) and 64.5% (Group B) at 3 years, respectively [Figure 3]. There was no significant difference between the OS of the 2 groups (log-rank test = 0.061).
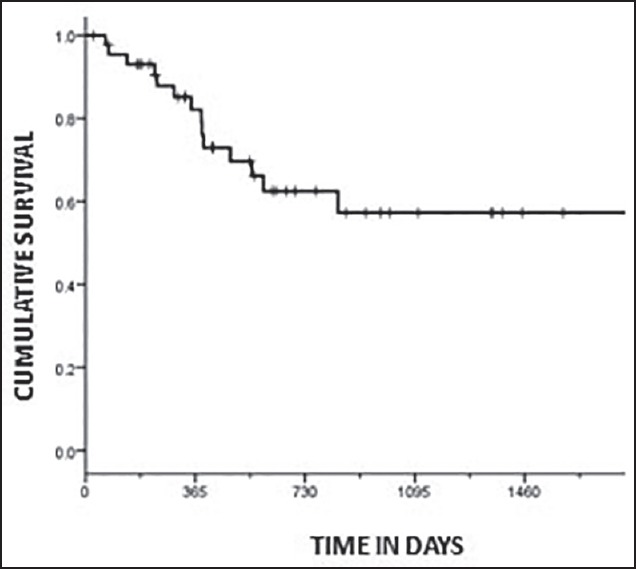
| Fig. 1 Kaplan-Meier plot showing disease-free survival for the whole group
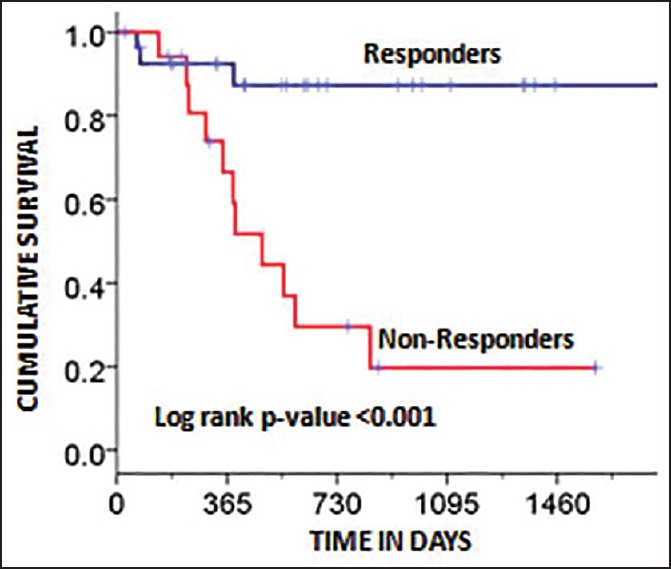
| Fig. 2 Kaplan-Meier plot showing disease-free survival factored by the two groups (responders and nonresponders). The log-rank P-value was significant (P < 0.001)
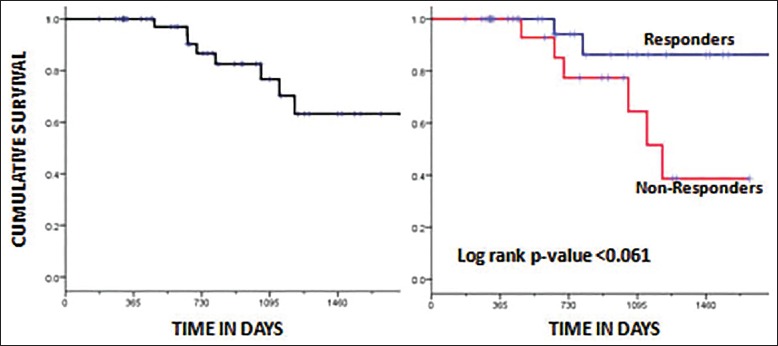
| Fig. 3 Kaplan-Meier plot showing overall survival for the whole group (a) and factored by the two groups (responders vs. nonresponders) (b). The log-rank P-value was not significant (P < 0.061).
Univariate and multivariate analysis
Univariate analysis was performed to know the relationship of the following factors with DFS: age (< 40 or >=40 years), sex, AJCC Stage (IIb and III vs. Iva and IVb), and response groups (responders vs. nonresponders). Response groups were found to be the only significant factors predicting DFS at 3 years (P = 0.002) [Table 2]. Similar results were seen on multivariate analysis with response groups being the only factor maintaining significance (P < 0.001).
Table 2
Prognostic variable analysis for nasopharyngeal carcinoma
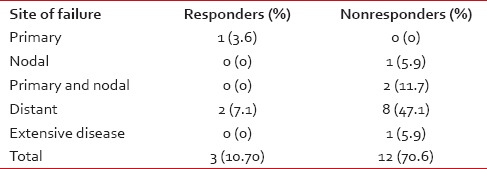
Sites of failure
In Group A, 25 of the 28 (89.3%) patients were controlled. Failure rate was 10.7% with one (3.6%) patient having a primary recurrence while 2 (7.1%) had distant failure.
In Group B, 5 of the 17 (29.3%) were disease-free. Failure rate was 70.7% with 1 (5.9%) patient having a nodal recurrence, 2 (11.8%) patients having a locoregional recurrence, 8 (47%) had only distant failure, and 1 (5.9%) patient had a locoregional recurrence with disseminated disease [Table 3].
Table 3
Patterns of failure in the two groups
DISCUSSION
Nasopharyngeal carcinomas are more radiosensitive than most other head and neck tumors making radiotherapy an important component of treatment. Several strategies using chemotherapy as a concomitant, neoadjuvant and adjuvant have been tried to improve control of this disease. Of these concomitant chemotherapy has shown the maximum benefit.[8] However, in spite of the advances local and distant failure continue to be a significant problem for these patients.
In contemporary series, DFS of 65-85% has been reported.[9,10,11,12] In our series, the DFS at 3 years was 57.3%. This disparity is because our series consists of patients with locally advanced disease (Stage IIb-IVb) while the above-mentioned series contained Stage I and IIa patients as well. This also represents the fact that there may be heterogeneity between subsets of patients and to improve outcomes, we have to identify prognostic and predictive markers.
A large number of prognostic factors have been identified such as stage group, neck node involvement, intracranial extension, cranial nerve involvement, and parapharyngeal involvement.[13] None of these have been able to predict outcome in nasopharyngeal carcinoma. This has generated a rejuvenated interest in identifying the biology of the disease by various studies.
Among the predictive factors studied the largest impact has been created by Epstein-Barr virus (EBV) titers and PET-CT. In a study, Lin et al. showed that OS and relapse-free survival were significantly lower among patients with pretreatment plasma EBV deoxyribonucleic acid (DNA) concentrations of at least 1500 copies/ml than among those with concentrations of < 1500 copies/ml. Patients with persistently detectable plasma EBV DNA had significantly worse OS and relapse-free survival than patients with undetectable EBV DNA 1 week after the completion of radiotherapy.[14] Chan et al. also demonstrated similar results but both studies used different cutoff values for pretreatment (4000 vs. 1500) and posttreatment (500 vs. 0) titers.[15] These studies demonstrated the utility of EBV titers albeit with limitations. Most importantly these studies highlight the interobserver variability of this approach based on the method used to detect the EBV DNA.
In another attempt, Kwong et al. used serial biopsies in a subset of 803 patients. In their study, they classified patients having the persistent disease as those whose biopsies were positive even after 12 weeks of treatment. They found that 29.3% of their patients had persistent disease. This cohort also had an inferior DFS compared to a group having negative biopsies before 12 weeks (47.4% vs. 71.8%). This subset of patients had a higher incidence of local and distant failure. They also found that two sessions of biopsies were warranted to reduce the false negative results at about 8-10 weeks.[16] This study was a landmark in the sense that it did identify the difference in remission patterns of the disease which may be due to the difference in biology of the disease between early and late responders. However, it also highlighted the fact that biopsy is an invasive procedure involving significant morbidity and anxiety to the patient. Importantly, it stressed on the high false negative rates of the biopsies. This may be due to the biopsy missing the active focus of the disease. Based on the results from our study, the 28.3% of their patients had persistent disease. This cohort also had an inferior DFS compared to a group having negative biopsies before 12 weeks (47.4% vs. 71.8%). This subset of patients had a higher incidence of local and distant failure. They also found that two sessions of biopsies were warranted to reduce the false negative results at about 8-10 weeks.[16] This study was a landmark in the sense that it did identify the difference in remission patterns of the disease which may be due to the difference in biology of the disease between early and late responders. However, it also highlighted the fact that biopsy is an invasive procedure involving significant morbidity and anxiety to the patient. Importantly, it stressed on the high false negative rates of the biopsies. This may be due to the biopsy missing the active focus of the disease. Based on the results from our study, the 28.3% cases that were missed by biopsy in Kwong's study may have been picked up by posttreatment PET-CT. This statement, however, needs further prospective validation. Furthermore, results from our study raise the possibility that there may be distant failure in the absence of locoregional disease and the PET-CT done at 10-12 weeks may help identify patients at greatest risk for this.
In their efforts to predict outcomes, Chan et al. studied a group of 65 patients with pretherapy PET-CT. They found that 15% patients failed distantly. Importantly, in their series, a pretherapy PET SUVmax (at the primary) of >12 predicted worse outcomes in the form of distant failures.[17]
In another study on pretherapy, PET-CT Lee et al. studied 41 patients. In their analysis, they showed that a pretherapy SUVmax >8 correlated with a worse DFS at 3 years (91% vs. 51%).[18]
Both these studies have reached similar conclusions but highlight the fact that SUVmax determination is a semi-quantitative method with the fallacy that there cannot be a single cutoff value. This also stresses that SUVmax may vary between institutions and patients. Thus, this method for prediction of response and hence, selection of patients for more aggressive therapy is not without its problems. Drawing analogy from the study by Kwong et al. this method may not be able to predict those patients who would have a complete response at the end of therapy.
In our study, we tried to analyze the utility of posttherapy PET-CT done at 10-12 weeks for its predictive value. We found that any uptake above the background activity at this time predicted worse DFS at 3 years (87.3% vs. 19.7%). The response groups were the only significant factors in univariate and multivariate analysis. We feel that any uptake at 10-12 weeks posttherapy may represent smoldering disease in the primary or nodes which has the capability to metastasize to distant areas as distant failure, which was the most common site of failure in our study. This may happen even in the absence of locoregional failure.
The timing of postradiation PET-CT has been a contentious issue. However, it has been shown that PET-CT performed before 10 weeks has a lower sensitivity due to the stunning effect of radiation therapy on vascular damage and cellular glucose transport. Based on this, we selected a time period of 10-12 weeks for performing the PET-CT.[4]
A Large number of trials using adjuvant therapy have failed to show benefit.[19,20,21] This may be due to the wrong selection of patients. In fact, it may be worth observing if the addition of adjuvant treatment in the above subset with residual uptake on PET-CT has the potential to improve the DFS and thus OS. In the light of emerging, literature on the use of EBV titers and PET-CT as predictive markers for nasopharyngeal carcinoma, it may also be worthwhile to conduct a prospective trial incorporating both these modalities and evaluating the predictive efficacy of both these modalities separately or together, the latter of which may help overcome the intrinsic problems of using either modality by itself.
CONCLUSION
Our study revealed that distant metastasis is a significant cause for failure in nasopharyngeal cancer and may often happen even in the absence of locoregional disease. The above analysis indicates that PET-CT performed 10-12 weeks after completion of chemoradiotherapy can be used as a method to evaluate response and maybe used as a tool to identify patients with carcinoma nasopharynx who may be at higher risk of distant failure. Further, this could be exploited to identify patients who may need treatment intensification. This needs to be validated prospectively.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Kaplan-Meier plot showing disease-free survival for the whole group

| Fig. 2 Kaplan-Meier plot showing disease-free survival factored by the two groups (responders and nonresponders). The log-rank P-value was significant (P < 0.001)

| Fig. 3 Kaplan-Meier plot showing overall survival for the whole group (a) and factored by the two groups (responders vs. nonresponders) (b). The log-rank P-value was not significant (P < 0.061).


 PDF
PDF  Views
Views  Share
Share

