Galactogram for Investigation of Pathological Nipple Discharge: A Forgotten Arrow in the Radiologists’ Quiver?
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 96-99
DOI: DOI: 10.4103/ijmpo.ijmpo_33_17
Abstract
Conventional X-ray galactogram (CG) is an underutilized procedure in modern breast imaging despite offering the highest spatial resolution among all modalities available for imaging of the breast ducts. The superior diagnostic performance of CG as compared to that of both conventional mammogram and high-resolution ultrasonography makes it a valuable imaging modality for the evaluation of pathological nipple discharge (PND). In addition, CG should always be considered in women with bloody nipple discharge but normal ultrasound and mammogram. CG also has an important role in the preoperative localization of intraductal lesions. CG may be especially useful in resource-restricted settings where breast magnetic resonance imaging is not readily available as it can be easily performed at any mammography facility without the need for additional equipment. In this article, we describe two cases of PND, one of benign and the other of malignant etiology, to demonstrate the value of CG in these cases. We also review the current literature and compare CG with other modalities used for imaging of ductal system of the breast.
Keywords
Breast imaging - cancer - galactogram - mammography - nipple discharge
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Conventional X-ray galactogram (CG) is an underutilized procedure in modern breast imaging despite offering the highest spatial resolution among all modalities available for imaging of the breast ducts. The superior diagnostic performance of CG as compared to that of both conventional mammogram and high-resolution ultrasonography makes it a valuable imaging modality for the evaluation of pathological nipple discharge (PND). In addition, CG should always be considered in women with bloody nipple discharge but normal ultrasound and mammogram. CG also has an important role in the preoperative localization of intraductal lesions. CG may be especially useful in resource-restricted settings where breast magnetic resonance imaging is not readily available as it can be easily performed at any mammography facility without the need for additional equipment. In this article, we describe two cases of PND, one of benign and the other of malignant etiology, to demonstrate the value of CG in these cases. We also review the current literature and compare CG with other modalities used for imaging of ductal system of the breast.
Keywords
Breast imaging - cancer - galactogram - mammography - nipple discharge
Introduction
A conventional X-ray galactogram (CG), also referred to as breast ductogram, is a specialized X-ray procedure used to view abnormalities in lactiferous ducts. CG has been traditionally considered the imaging modality of choice in the evaluation of pathological nipple discharge (PND). However, it has been largely replaced by high-resolution ultrasound (HR-USG) as the initial imaging modality in patients with nipple discharge as it is semi-invasive and requires ionizing radiation. However, CG still offers the best spatial resolution among the existing imaging modalities and has a fairly high sensitivity.
In this article, we demonstrate the usefulness of CG in clinching the diagnosis in two cases of PND. We also present a brief overview of the literature and compare CG with other imaging modalities.
Case Reports
Case 1
A 44-year-old woman presented with a 2-week history of intermittent spontaneous painless discharge from the right nipple. Physical examination did not reveal any mass or axillary lymphadenopathy. Hemorrhagic discharge from one duct was observed in the right breast on compression during mammography.
A CG was performed after injecting approximately 3.5 cc of iodinated contrast medium into the discharging duct, and standard mediolateral oblique (MLO) and craniocaudal (CC) views were obtained using full-field digital mammography [Figure 1]. The galactogram showed focal-on-diffuse duct irregularity in the upper and outer quadrants of the right breast along with faint micro filling defects, periductal extravasation, and areas of irregular ductal narrowing associated with sacculations and microcysts in the distal subsegmental ducts. Abrupt cutoff of segmental duct was seen immediately inferior to the first branching point of the main duct. Using the Galactogram Imaging Classification System (GICS), the findings were classified as GICS 5, indicating a high suspicion of malignancy. Targeted USG did not reveal any focal lesion or segmental duct ectasia. Cytology of the nipple discharge showed malignant cells. The patient was referred to the oncology services for staging and further management.
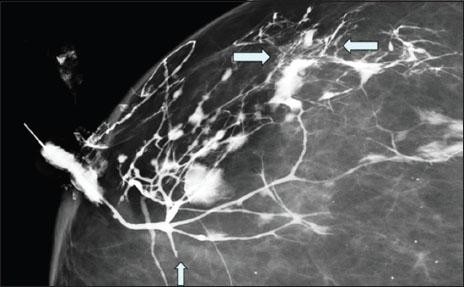
|?Figure.1Magnified craniocaudal view of galactogram of Case 1 showing diffuse duct irregularities in the upper and outer quadrants of the right breast, with particularly prominent findings in a small area of peripheral portion of the breast (between horizontal arrows). Key features include faint microdefects with ductal wall irregularities, periductal extravasation of contrast material, and areas of ductal narrowing and sacculation located in distal subsegmental ducts with microcysts. Abrupt cutoff of segmental duct is seen immediately distal to the first branching point of the main duct without any filling defect
Case 2
A 39-year-old woman presented with a 1-month-long history of persistent nipple discharge from the left breast. On examination, serosanguinous discharge was expressed from a single duct. No mass was palpable. Conventional mammography revealed no abnormality [Figure 2]a. Galactography was performed after injection of approximately 2 cc of nonionic iodinated contrast media through a 30G plastic cannula inserted in the culprit duct. CG showed a dilated major duct in the subareolar region with at least two well-defined filling defects. The smaller filling defect showed clear concave termination and a subtle extension to one of the subsegmental ducts [Figure 2]b. These findings were consistent with a suspicious pathology (GICS 4). USG showed an echogenic elongated nodular mass within a dilated subareolar duct. The mass showed internal vascularity on power Doppler [Figure 2]c. The smaller filling defect was not localized on USG. Duct excision was done and histopathology revealed multiple duct papillomas.
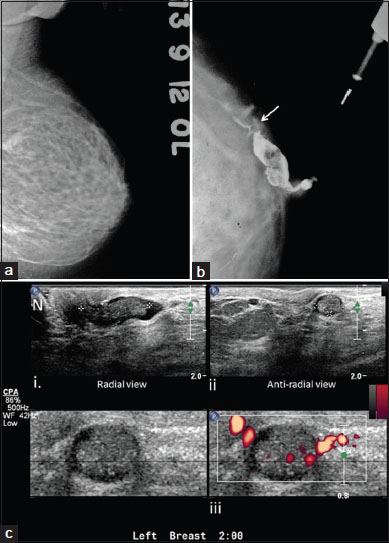
|?Figure.2Mammogram, galactogram, and high-resolution USG of the left breast of a woman with pathological nipple discharge. (a) CC view of the left breast shows no obvious abnormality; (b) Galactogram, CC view reveals dilated duct with intraluminal filling defect in retroareolar tissue in the superior quadrant of the left breast. Notice the small filling defect extending in the subsegmental duct in superior quadrant (arrow); (c) USG shows dilated duct with echogenic intraluminal mass in radial (i) and antiradial (ii) views in the subareolar region. Notice nipple (N). Power Doppler image shows vascularity within the mass (iii). USG: Ultrasound; CC: Craniocaudal
Discussion
Nipple discharge has a prevalence of about 3%?10% and is the third most common breast-related complaint after mass and breast pain.[1],[2] PND is defined as persistent, spontaneous, nonlactational, unilateral, and single-duct discharge.[2] PND is caused most commonly by benign lesions such as duct papillomas. Nearly 6%?20?ses of PND are due to underlying malignancy such as ductal carcinoma?in situ?(DCIS) and invasive intraductal carcinoma. Risk of malignancy is higher in women of age> 50 years and in cases of hemorrhagic discharge.[2],[3] Therefore, proper evaluation of every case of PND is critical.
The imaging modalities that are most commonly used in cases of PND are mammography, HR-USG, CG, and contrast-enhanced magnetic resonance imaging (CE-MRI). The gold standard for diagnosis in PND is major duct excision (MDE) or surgical pathology.[4]
Conventional galactogram is still considered the gold standard of ductal imaging by some authors.[5],[6] CG is an X-ray mammography-based procedure. It is performed by cannulating the abnormal duct with a thin plastic cannula and injecting 2?5 mL iodinated nonionic contrast media to opacify the ductal system. After contrast injection, standard MLO and CC mammograms are taken.
The main indication for performing a galactogram is PND. Findings that are suggestive of a benign pathology include duct ectasia, macrodefects with concave meniscus, and cystic changes with ductal communication [Case 2, [Figure 2]. Abrupt or irregular duct obstruction, duct wall irregularity, ductal stenosis and sacculations, micro filling defects, and contrast extravasation in a woman> 50 years of age are suggestive of a malignant etiology [2] [Case 1, [Figure 1]. Duct obstruction due to benign pathology is more often restricted to the periareolar region and involves the main or segmental ducts whereas carcinomas are more commonly located in the peripheral portions of the breast. GICS classifies CG findings into five categories in an ascending order of suspicion for malignancy.[7]
CG has moderate negative predictive value ranging from 26% to 63% for diagnosis of ductal lesions.[4],[8] CG has several disadvantages. It is semi-invasive and involves compression of the breast tissue and radiation exposure. Failure to cannulate the duct can result in incomplete study in up to 15% of cases.[1],[2] CG also lacks specificity and has a low positive predictive value in the diagnosis of ductal lesions.[4] While some authors have demonstrated a statistically significant correlation between histopathology and CG findings,[2] others have found that CG fares poorly in differentiating between benign and malignant lesions.[8],[9] Combined use of digital breast tomosynthesis and CG may improve its diagnostic performance.[10]
Galactography plays an important role in the localization of breast neoplasms and in guiding the choice and extent of surgical therapy.[11],[12] Accurate localization allows focused ductal excision. The use of CG has been shown to improve the diagnostic yield of surgical biopsy and MDE.[3],[13] CG-guided vacuum-assisted breast biopsy is also a potential diagnostic tool.[14]
Mammogram has a very low sensitivity (20%?25%) for PND.[1] A negative mammogram does not exclude malignant pathology in patients with PND [9] as it can be negative in as many as 94% of cases of PND.[2]
HR-USG is replacing CG as the initial investigation of choice for PND in many institutes. USG has moderate sensitivity (65%) and moderate-to-high specificity (75%?85%) for the diagnosis of ductal lesions.[1] However, USG may be negative in up to 80?ses of PND of a malignant etiology.[15] In Case 1, USG failed to localize any lesion, while in Case 2, it failed to localize the smaller papilloma that was readily seen on CG. Therefore, USG may not be reliable for excluding malignant causes of nipple discharge. USG is a valuable tool as a ?second-look? modality after a suspicious area is identified on mammography, CG, or MRI. CG is superior to USG for diagnosis of intraductal pathology.[9]
Contrast-enhanced MR mammography has a high sensitivity for detection of pathology in cases of nipple discharge; however, it has a high false positive rate.[16] MRI is free of ionizing radiation and offers simultaneous imaging of the ductal and extraductal pathology in a three-dimensional (3D) view. The patterns of contrast enhancement on CE-MRI have been shown to correlate with different ductal pathologies.[8] Mass-like enhancement is more commonly seen in duct papillomas whereas segmental enhancement is more commonly seen with DCIS. Various methods may be employed for ductal imaging on MRI. Injection of gadolinium-based contrast media into the pathological duct and obtaining postinjection T1-weighted 3D sequences provides images analogous to that on CG. However, this technique is still experimental.[16] Another technique employs 3D heavily T2-weighted fat-suppressed technique for imaging of dilated ducts in a way that is analogous to MR cholangiopancreaticography.[17]
There are very few guidelines for imaging evaluation of PND. The Institute for Clinical Systems Improvement guidelines recommends the use of mammogram and HR-USG as the initial imaging modality in patients with PND. In cases that are negative on conventional imaging studies, CG or CE-MRI may be done.[18] It is pertinent to note that CG does not require any additional setup beyond a standard mammography machine. On the other hand, the use of breast MR is limited by high cost and limited availability of equipment and specialized breast coils. The European Society of Breast Cancer Specialists does not recommend the routine use of MRI in PND. It suggests that in countries where CG is considered a routine test for PND, MRI should be considered only if CG fails for technical reasons or if the patient refuses the procedure.[19]
Conclusion
CG is fairly simple to perform yet is an underused radiological technique in the current clinical practice. It is especially suitable for resource-poor settings due to poor access to MRI as CG is superior to both conventional mammogram and HR-USG in the diagnosis of intraductal lesions. It has the added advantage of superior localization which is useful for planning of biopsy and surgery. This article underlines the usefulness of galactogram in clinical practice and highlights the need to bring it back to the forefront of imaging of PND in all diagnostic mammography departments.
Conflict of Interest
There are no conflicts of interest.
References
- ippa N, Hurtevent-Labrot G, Ferron S, Boisserie-Lacroix M.?Nipple discharge: The role of imaging. Diagn Interv Imaging 2015; 96: 1017-32
- ern?-Serna JD, Torres-Ales C, Bern?-Mestre JD, Polo L.?et al.?Role of galactography in the early diagnosis of breast cancer. Breast Care (Basel) 2013; 8: 122-6
- amont JP, Dultz RP, Kuhn JA, Grant MD, Jones RC.?Galactography in patients with nipple discharge. Proc (Bayl Univ Med Cent) 2000; 13: 214-6
- orrogh M, Morris EA, Liberman L, Borgen PI, King TA.?The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol 2007; 14: 3369-17
- ab?r L, Dean PB, P?ntek Z.?Galactography: The diagnostic procedure of choice for nipple discharge. Radiology 1983; 149: 31-8
- erris-James DM, Iuanow E, Mehta TS, Shaheen RM, Slanetz PJ.?Imaging approaches to diagnosis and management of common ductal abnormalities. Radiographics 2012; 32: 1009-30
- ern?-Serna JD, Torres-Al?s C, Bern?-Mestre JD, Sola-P?rez J, Canteras-Jordana M.?Galactography: An application of the Galactogram Imaging Classification System (GICS). Acta Radiol 2010; 51: 128-36
- anganaro L, D'Ambrosio I, Gigli S, Di PastenaF, Giraldi G, Tardioli S.?et al.?Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: A comparison with galactography. Biomed Res Int 2015; 2015: 806368
- lum KS, Rubbert C, Antoch G, Mohrmann S, Obenauer S.?Diagnostic accuracy of abnormal galactographic and sonographic findings in the diagnosis of intraductal pathology in patients with abnormal nipple discharge. Clin Imaging 2015; 39: 587-91
- Cohen Y.?Conventional ductography combined with digital breast tomosynthesis for imaging of pathologic nipple discharge. AJR Am J Roentgenol 2016; 206: W44
- Scheurlen K, Schnitzer A, Krammer J, Kaiser C, Sch?nberg SO, Wasser K.?Value of galactography for the diagnostic work-up of pathological nipple discharge in multimodal breast diagnostics: Part 1: An online survey among German breast care centers. Radiologe 2014; 54: 63-7
- Dinkel HP, Trusen A, Gassel AM, Rominger M, Lourens S, M?ller T.?et al.?Predictive value of galactographic patterns for benign and malignant neoplasms of the breast in patients with nipple discharge. Br J Radiol 2000; 73: 706-14
- Van ZeeKJ, Ortega P?rezG, Minnard E, Cohen MA. Van KJ, Ortega P?rez G, Minnard E, Cohen MA?Preoperative galactography increases the diagnostic yield of major duct excision for nipple discharge. Cancer 1998; 82: 1874-80
- Reiner CS, Helbich TH, Rudas M, Ponhold L, Riedl CC, Kropf N.?et al.?Can galactography-guided stereotactic, 11-gauge, vacuum-assisted breast biopsy of intraductal lesions serve as an alternative to surgical biopsy?. Eur Radiol 2009; 19: 2827-85
- Rissanen T, Reinikainen H, Apaja-Sarkkinen M.?Breast sonography in localizing the cause of nipple discharge: Comparison with galactography in 52 patients. J Ultrasound Med 2007; 26: 1031-9
- Y?cesoy C, Ozt?rk E, Ozer Y, Edg?er T, Hekimoglu B.?Conventional galactography and MR contrast galactography for diagnosing nipple discharge: Preliminary results. Korean J Radiol 2008; 9: 426-31
- Nicholson BT, Harvey JA, Patrie JT, Mugler 3rd JP.?3D-MR ductography and contrast-enhanced MR mammography in patients with suspicious nipple discharge; a feasibility study. Breast J 2015; 21: 352-62
- Institute for Clinical Systems Improvement. Minneapolis: Institute for Clinical Systems Improvement; January, 2012. Available from: https://www.icsi.org/_asset/v9l91q/DxBrDis.pdf. [Last accessed on 2017 Feb 14].
- Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ.?et al.?Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer 2010; 46: 1296-316
Address for correspondence
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure.1Magnified craniocaudal view of galactogram of Case 1 showing diffuse duct irregularities in the upper and outer quadrants of the right breast, with particularly prominent findings in a small area of peripheral portion of the breast (between horizontal arrows). Key features include faint microdefects with ductal wall irregularities, periductal extravasation of contrast material, and areas of ductal narrowing and sacculation located in distal subsegmental ducts with microcysts. Abrupt cutoff of segmental duct is seen immediately distal to the first branching point of the main duct without any filling defect

|?Figure.2Mammogram, galactogram, and high-resolution USG of the left breast of a woman with pathological nipple discharge. (a) CC view of the left breast shows no obvious abnormality; (b) Galactogram, CC view reveals dilated duct with intraluminal filling defect in retroareolar tissue in the superior quadrant of the left breast. Notice the small filling defect extending in the subsegmental duct in superior quadrant (arrow); (c) USG shows dilated duct with echogenic intraluminal mass in radial (i) and antiradial (ii) views in the subareolar region. Notice nipple (N). Power Doppler image shows vascularity within the mass (iii). USG: Ultrasound; CC: Craniocaudal
References
- ippa N, Hurtevent-Labrot G, Ferron S, Boisserie-Lacroix M.?Nipple discharge: The role of imaging. Diagn Interv Imaging 2015; 96: 1017-32
- ern?-Serna JD, Torres-Ales C, Bern?-Mestre JD, Polo L.?et al.?Role of galactography in the early diagnosis of breast cancer. Breast Care (Basel) 2013; 8: 122-6
- amont JP, Dultz RP, Kuhn JA, Grant MD, Jones RC.?Galactography in patients with nipple discharge. Proc (Bayl Univ Med Cent) 2000; 13: 214-6
- orrogh M, Morris EA, Liberman L, Borgen PI, King TA.?The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol 2007; 14: 3369-17
- ab?r L, Dean PB, P?ntek Z.?Galactography: The diagnostic procedure of choice for nipple discharge. Radiology 1983; 149: 31-8
- erris-James DM, Iuanow E, Mehta TS, Shaheen RM, Slanetz PJ.?Imaging approaches to diagnosis and management of common ductal abnormalities. Radiographics 2012; 32: 1009-30
- ern?-Serna JD, Torres-Al?s C, Bern?-Mestre JD, Sola-P?rez J, Canteras-Jordana M.?Galactography: An application of the Galactogram Imaging Classification System (GICS). Acta Radiol 2010; 51: 128-36
- anganaro L, D'Ambrosio I, Gigli S, Di PastenaF, Giraldi G, Tardioli S.?et al.?Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: A comparison with galactography. Biomed Res Int 2015; 2015: 806368
- lum KS, Rubbert C, Antoch G, Mohrmann S, Obenauer S.?Diagnostic accuracy of abnormal galactographic and sonographic findings in the diagnosis of intraductal pathology in patients with abnormal nipple discharge. Clin Imaging 2015; 39: 587-91
- Cohen Y.?Conventional ductography combined with digital breast tomosynthesis for imaging of pathologic nipple discharge. AJR Am J Roentgenol 2016; 206: W44
- Scheurlen K, Schnitzer A, Krammer J, Kaiser C, Sch?nberg SO, Wasser K.?Value of galactography for the diagnostic work-up of pathological nipple discharge in multimodal breast diagnostics: Part 1: An online survey among German breast care centers. Radiologe 2014; 54: 63-7
- Dinkel HP, Trusen A, Gassel AM, Rominger M, Lourens S, M?ller T.?et al.?Predictive value of galactographic patterns for benign and malignant neoplasms of the breast in patients with nipple discharge. Br J Radiol 2000; 73: 706-14
- Van ZeeKJ, Ortega P?rezG, Minnard E, Cohen MA. Van KJ, Ortega P?rez G, Minnard E, Cohen MA?Preoperative galactography increases the diagnostic yield of major duct excision for nipple discharge. Cancer 1998; 82: 1874-80
- Reiner CS, Helbich TH, Rudas M, Ponhold L, Riedl CC, Kropf N.?et al.?Can galactography-guided stereotactic, 11-gauge, vacuum-assisted breast biopsy of intraductal lesions serve as an alternative to surgical biopsy?. Eur Radiol 2009; 19: 2827-85
- Rissanen T, Reinikainen H, Apaja-Sarkkinen M.?Breast sonography in localizing the cause of nipple discharge: Comparison with galactography in 52 patients. J Ultrasound Med 2007; 26: 1031-9
- Y?cesoy C, Ozt?rk E, Ozer Y, Edg?er T, Hekimoglu B.?Conventional galactography and MR contrast galactography for diagnosing nipple discharge: Preliminary results. Korean J Radiol 2008; 9: 426-31
- Nicholson BT, Harvey JA, Patrie JT, Mugler 3rd JP.?3D-MR ductography and contrast-enhanced MR mammography in patients with suspicious nipple discharge; a feasibility study. Breast J 2015; 21: 352-62
- Institute for Clinical Systems Improvement. Minneapolis: Institute for Clinical Systems Improvement; January, 2012. Available from: https://www.icsi.org/_asset/v9l91q/DxBrDis.pdf. [Last accessed on 2017 Feb 14].
- Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ.?et al.?Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer 2010; 46: 1296-316


 PDF
PDF  Views
Views  Share
Share

