Gefitinib-induced skin ulceration in metastatic adenocarcinoma lung
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 109-110
DOI: DOI: 10.4103/0971-5851.133736
Abstract
We report a case of gefitinib-induced skin ulceration in a 50-year-old female with metastatic adenocarcinoma of lung who developed this adverse effect 2 weeks following initiation of gefitinib at a dose of 250 mg/day. The ulcer improved with stopping gefitinib for 2 weeks and also addition of topical steroids and antibiotics. We are reporting this case to create awareness among treating oncologists of this adverse effect and also prompt interruption of therapy and topical steroids/antibiotics is useful to treat this adverse event.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We report a case of gefitinib-induced skin ulceration in a 50-year-old female with metastatic adenocarcinoma of lung who developed this adverse effect 2 weeks following initiation of gefitinib at a dose of 250 mg/day. The ulcer improved with stopping gefitinib for 2 weeks and also addition of topical steroids and antibiotics. We are reporting this case to create awareness among treating oncologists of this adverse effect and also prompt interruption of therapy and topical steroids/antibiotics is useful to treat this adverse event.
INTRODUCTION
The Epidermal growth factor receptor (EGFR) is over expressed in various solid malignancies including non small cell lung cancer (NSCLC). Hence EGFR inhibitors - gefitinib and erlotinib are used in advanced NSCLC. However, they are associated with a dermatologic side effects, which can occasionally be responsible for discontinuation of the EGFR inhibitors. Hence, we report a case of metastatic adenocarcinoma of lung who developed skin ulceration with gefitinib and responded to interruption of the drug and early intervention.
CASE REPORT
The present case report is about a 50-year-old female patient who had been diagnosed as having lung adenocarcinoma with multiple bone metastases was initiated on gefitinib therapy at an oral dose of 250 mg/d. She had a positive epidermal growth factor receptor (EGFR) mutation status. After 2 weeks of initiating therapy, the patient presented with ulcer over the palm [Figure 1]. The ulcers improved with stopping gefitinib for 2 weeks and also with the addition of topical steroids and antibiotics.
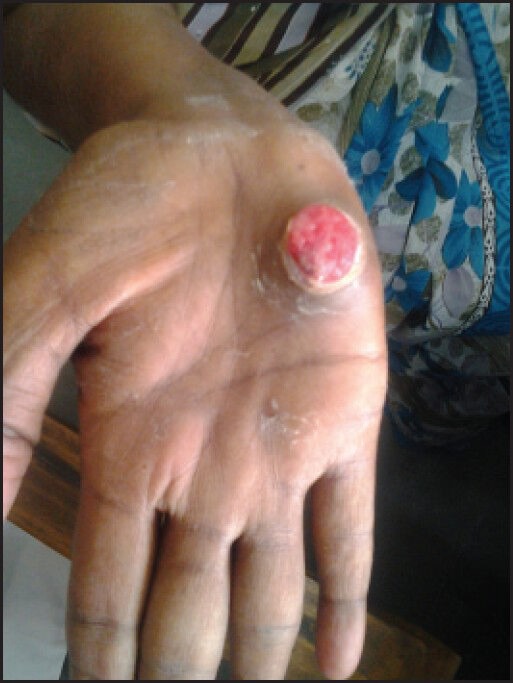
| Figure 1:Ulcer over the palm with granulation tissue in a woman on gefitinib
DISCUSSION
Non-small-cell lung cancer (NSCLC) with sensitive mutations of the EGFR is highly responsive to gefitinib. Gefitinib is a small molecule tyrosine kinase inhibitor (TKI) of EGFR.[1] Since 2004, it was clear that a substantial proportion of NSCLC obtaining objective response when treated with gefitinib harboring activating mutations in the EGFR gene.[2] The occurrence of skin disorders (dry skin and acneiform rash) is explained by the fact that EGFR is also expressed in the basal layer of the skin; inhibition of the receptor will disturb normal biology and result in skin rash.[3,4] Skin rash is notorious as an adverse event of EGFR-TKI and is noted in up to two-thirds of patients receiving any of these agents although severe in only 5-10% who can develop pyogenic granuloma like lesions. Very rarely the cutaneous inflammation is so pronounced that skin necrosis with black eschar formation and ulceration is seen.[5] The cutaneous side-effects are treated with topical steroids and antibiotics with interruption of treatment for 2-4 weeks as in our case. Our patient responded to the above treatment with break in treatment for 2 weeks.[6]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United States food and drug administration drug approval summary: Gefitinib ZD1839; Iressa) tablets. Clin Cancer Res 2004;10:1212-8.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
- Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: Histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 2002;20:110-24.
- Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol 2004;151:238-41.
- Lee MW, Seo CW, Kim SW, Yang HJ, Lee HW, Choi JH, et al. Cutaneous side effects in non-small cell lung cancer patients treated with Iressa (ZD1839), an inhibitor of epidermal growth factor. Acta Derm Venereol 2004;84:23-6.
- Matheis P, Socinski MA, Burkhart C, Warren S, Thomas NE. Treatment of gefitinib-associated folliculitis. J Am Acad Dermatol 2006;55:710-3.

| Figure 1:Ulcer over the palm with granulation tissue in a woman on gefitinib
References
- Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United States food and drug administration drug approval summary: Gefitinib ZD1839; Iressa) tablets. Clin Cancer Res 2004;10:1212-8.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
- Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: Histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 2002;20:110-24.
- Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol 2004;151:238-41.
- Lee MW, Seo CW, Kim SW, Yang HJ, Lee HW, Choi JH, et al. Cutaneous side effects in non-small cell lung cancer patients treated with Iressa (ZD1839), an inhibitor of epidermal growth factor. Acta Derm Venereol 2004;84:23-6.
- Matheis P, Socinski MA, Burkhart C, Warren S, Thomas NE. Treatment of gefitinib-associated folliculitis. J Am Acad Dermatol 2006;55:710-3.


 PDF
PDF  Views
Views  Share
Share

