Germ Cell Tumors in Children: A Retrospective Review of a 04-Year Single-Center Experience
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(03): 269-277
DOI: DOI: 10.1055/s-0044-1796674
Abstract
Introduction Pediatric germ cell tumors (GCTs) are rare neoplasms that can be benign or malignant and occur in children and adolescents. They can arise from germ cells in the extragonadal or gonadal sites and have numerous histologic subtypes. The International Germ Cell Cancer Cooperative Group classification is used to guide treatment, with most children experiencing excellent overall survival (OS). Chemotherapy is historically recommended for all malignant GCTs. Nevertheless, surgery and observation alone may be sufficient for stage I gonadal GCTs. Using chemotherapy can lead to successful salvage of relapses..
Objectives Our institute has conducted a study with the objective of evaluating a group of 97 patients diagnosed with GCTs, who received treatment within the past 4 years.
Materials and Methods From January 2018 to April 2022, a total of 97 pediatric patients diagnosed with GCTs underwent surgical treatment at Indus Hospital & Health Network. The diagnosis was established by considering clinical features, tumor marker levels, imaging, and histology. Treatment was determined based on the risk stratification utilizing the United Kingdom Children's Cancer Study Group GC 2005–04 protocol. Patients classified as LR (low-risk) received chemotherapy only if a recurrence occurred after the initial surgery. On the other hand, IR (intermediate-risk) and HR (high-risk) patients received four and six cycles of JEB (chemotherapy regimen), respectively, followed by surgery. Recurrence was closely monitored through suspicion, tumor marker levels, or imaging. Patients experiencing a recurrence after JEB chemotherapy were treated with TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy and subsequently underwent surgery.
Results In this retrospective study, a group of 97 patients diagnosed with GCTs was analyzed. The cohort included 59 gonadal tumors and 38 extragonadal tumors. The most common histopathological types observed were yolk sac tumor and dysgerminoma. Out of the patients, 33 (34%) were classified as HR, 35 (36.1%) as IR, and 29 (29.9%) as LR. Among the patients, 16 experienced recurrence, while the remaining 90 patients (92.8%) were alive at the time of the analysis. The study determined the 5-year event-free survival (EFS) rate as 83.5%-and the OS rate as 92.8%. The presence of residual disease was found to be the only significant factor that influenced EFS.
Conclusion To manage GCTs in children, a multidisciplinary approach involving surgeons, oncologists, and radiation therapists is required. Surgery and chemotherapy have improved outcomes for children with GCTs, but personalized treatment planning is crucial. With advancements in pediatric oncology care, the prognosis for children with GCT in Pakistan has improved, providing hope for better outcomes in the future.
Keywords
germ cell tumors (GCT) - children - gonadal - yolk sac tumor - overall survival - event-free survivalPatient Consent
Informed patient consent was obtained for this study.
Publication History
Article published online:
24 February 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Second Malignant Neoplasms in Children and Adolescents Treated for Blood Malignancies and Solid Tumors: A Single-Center Experience of 15 YearsNikolaos Katzilakis, Indian Journal of Medical and Paediatric Oncology, 2018
- Supratentorial and Infratentorial Approaches to Pineal Surgery: A Database AnalysisDavid M. Rosenberg, Journal of Neurological Surgery Part B: Skull Base
- Mediastinal Germ Cell TumorsAlan Sandler, Seminars in Respiratory and Critical Care Medicine, 1997
- Mediastinal Germ Cell TumorsAlan Sandler, Semin Respir Crit Care Med, 1997
- Peculiarities of Yolk Sac Tumor in Head and Neck: A Case Report and Literature ReviewK Devaraja, Indian Journal of Medical and Paediatric Oncology, 2018
- Tooth number abnormality: from bench to bedside<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Genomic alterations in oral multiple primary cancers<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Resetting histone modifications during human prenatal germline development<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- The impact of interval between surgery and postoperative radiotherapy in major salivary gland carcinoma<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Taking inclusion in peer review to a new level: Kids as reviewers for scientific manuscripts<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Introduction Pediatric germ cell tumors (GCTs) are rare neoplasms that can be benign or malignant and occur in children and adolescents. They can arise from germ cells in the extragonadal or gonadal sites and have numerous histologic subtypes. The International Germ Cell Cancer Cooperative Group classification is used to guide treatment, with most children experiencing excellent overall survival (OS). Chemotherapy is historically recommended for all malignant GCTs. Nevertheless, surgery and observation alone may be sufficient for stage I gonadal GCTs. Using chemotherapy can lead to successful salvage of relapses..
Objectives Our institute has conducted a study with the objective of evaluating a group of 97 patients diagnosed with GCTs, who received treatment within the past 4 years.
Materials and Methods From January 2018 to April 2022, a total of 97 pediatric patients diagnosed with GCTs underwent surgical treatment at Indus Hospital & Health Network. The diagnosis was established by considering clinical features, tumor marker levels, imaging, and histology. Treatment was determined based on the risk stratification utilizing the United Kingdom Children's Cancer Study Group GC 2005–04 protocol. Patients classified as LR (low-risk) received chemotherapy only if a recurrence occurred after the initial surgery. On the other hand, IR (intermediate-risk) and HR (high-risk) patients received four and six cycles of JEB (chemotherapy regimen), respectively, followed by surgery. Recurrence was closely monitored through suspicion, tumor marker levels, or imaging. Patients experiencing a recurrence after JEB chemotherapy were treated with TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy and subsequently underwent surgery.
Results In this retrospective study, a group of 97 patients diagnosed with GCTs was analyzed. The cohort included 59 gonadal tumors and 38 extragonadal tumors. The most common histopathological types observed were yolk sac tumor and dysgerminoma. Out of the patients, 33 (34%) were classified as HR, 35 (36.1%) as IR, and 29 (29.9%) as LR. Among the patients, 16 experienced recurrence, while the remaining 90 patients (92.8%) were alive at the time of the analysis. The study determined the 5-year event-free survival (EFS) rate as 83.5%-and the OS rate as 92.8%. The presence of residual disease was found to be the only significant factor that influenced EFS.
Conclusion To manage GCTs in children, a multidisciplinary approach involving surgeons, oncologists, and radiation therapists is required. Surgery and chemotherapy have improved outcomes for children with GCTs, but personalized treatment planning is crucial. With advancements in pediatric oncology care, the prognosis for children with GCT in Pakistan has improved, providing hope for better outcomes in the future.
Keywords
germ cell tumors (GCT) - children - gonadal - yolk sac tumor - overall survival - event-free survivalIntroduction
Pediatric germ cell tumors (GCTs) are rare tumors. GCTs originate from aberrant differentiation of germ cells and encompass a heterogeneous collection of neoplasms, exhibiting notable variations in histological characteristics and site of occurrence.[1] [2] Children and adolescents can exhibit both benign and malignant GCTs, and the incidence rates of these tumors vary depending on their age.[3]
The overall incidence of GCTs in children up to 15 years of age can be estimated at 0.9 per 100,000 children.[1] [3] In infants, majority are benign GCTs, with the most common site being sacrococcygeal. GCT accounts for 2 to 3%-of all pediatric malignancies.[3] Out of all pediatric GCTs, approximately 60%-originate from extragonadal sites, such as the mediastinum, pineal and sacrococcygeal regions, and retroperitoneum. On the other hand, the remaining 40%-of cases are attributed to GCTs arising from the gonadal sites, specifically the ovary and testis.[4] [5] GCTs are the most frequently occurring tumors in the gonads among children and adolescents.[6] While GCTs may exhibit notable variations depending on their anatomical location, they possess a significant degree of similarity and are generally regarded as a cohesive group.[1] GCTs shows a wide range of histologic subtypes. The histologic characteristics of each subtype are not influenced by the clinical presentation. However, the behavior of the tumor and its biological attributes vary based on factors such as the site of origin, stage of the tumor, and age of the patient.[7]
The International Germ Cell Cancer Cooperative Group (IGCCCG) classification is globally employed to identify patients at high risk (HR) and provide guidance for first-line treatment.[8] As per this classification, patients diagnosed with non-germinomatous GCTs or those with elevated levels of tumor markers are categorized as HR individuals.
The majority of children diagnosed with GCTs achieve remarkable overall survival (OS) rates, which has consequently resulted in a reduction in the extent of chemotherapy administered.[9] In the past, chemotherapy was typically advised for all cases of malignant GCTs. Nevertheless, research has demonstrated that stage I gonadal GCTs can be effectively managed with surgical intervention and close monitoring, without the need for additional therapy in many patients. Even in cases of relapse, chemotherapy has proven highly successful in salvaging the patients.[10] [11]
To gain deeper insights into the clinical features and treatment approaches for pediatric GCTs, we conducted a comprehensive analysis of a cohort consisting of 97 patients with GCTs who were treated at our institution over the past 4 years.
Materials and Methods
During the period spanning from January 2018 to April 2022, a group of 97 pediatric patients (16 years old or younger) diagnosed with GCTs received surgical treatment at the Indus Hospital & Health Network. The hospital's electronic database was utilized to gather comprehensive information including clinical records, radiological data, laboratory results, and pathological findings.
The diagnosis of GCTs was established by considering various factors, including clinical manifestations, elevated levels of tumor markers (such as serum α-fetoprotein [AFP] and β human chorionic gonadotropin [β-HCG]), imaging studies, and histological analysis. Each patient underwent contrast-enhanced computed tomography scans of the chest, abdomen, and pelvis. For pelvic tumors, magnetic resonance imaging of the pelvis with contrast was performed. Additionally, bone scintigraphy was conducted for staging purposes.
The United Kingdom Children's Cancer Study Group GC 2005–04 protocol was employed for both staging and treatment purposes. Patients were categorized into three risk groups: low risk (LR), intermediate risk (IR), and HR, based on their stage and prognostic factors. Patients classified as HR included those with AFP levels greater than 10,000 ng/mL, stage IV disease (excluding testis < 5 years and all germinomas), or stage II to IV mediastinal tumors. For stage I tumors (LR), chemotherapy was administered only if there was disease recurrence following surgery. IR and HR patients received first-line chemotherapy consisting of four and six cycles of JEB (carboplatin 600 mg/m2 on day 2, etoposide 120 mg/m2 for 3 days, and bleomycin 15 mg/m2 on day 3), respectively, followed by surgical intervention. Tumor markers were monitored to assess treatment response. After completing the initial treatment, all patients underwent long-term surveillance to detect any recurrence of the disease. In cases where patients experienced a recurrence after JEB chemotherapy, they received four cycles of second-line chemotherapy known as TIP (paclitaxel 175 mg/m2 on day 1, ifosfamide 1500 mg/m2 from day 2 to 6 , cisplatin 20 mg/m2 from day 2 to 6) followed by surgery.
The patients were closely monitored to assess their event-free survival (EFS) and OS rates. An “event” was defined as disease relapse/progression or death from any cause. EFS was determined by calculating the time from the start of treatment to the occurrence of the event, while OS was calculated from the start of treatment to the date of the last follow-up or date of death. In survival analysis, all patients were considered until the date of the last follow-up or until April 30, 2022, whichever came earlier.
Primary Outcome
In this study, EFS and OS were the primary outcomes.
Secondary Outcome
Most of the tumor recurrences occurred in the HR group of GCT and was found as an additional information.
Inclusion Criteria
Patients with biopsy-proven extracranial GCTs within the age range of 0 to 16 years.
Patients who received surgical treatment outside of our hospital were also included.
Exclusion Criteria
Patients older than 16 years were excluded.
Patients with intracranial GCTs.
Patients with refractory or progressive extracranial GCT treated outside of our institution.
Statistical Analysis
The data analysis was performed using the Statistical Package for Social Sciences (SPSS; IBM, version 24.0, Armonk, New York, United States). For age, AFP, HCG, and lactate dehydrogenase (LDH) variables, the median (interquartile range) was calculated as the data did not follow a normal distribution. Frequencies and percentages were calculated for sex, histology, stage of the disease, presence of metastatic disease, initial treatment characteristics, recurrence, and outcomes. The frequency distributions along with corresponding percentages were also calculated for the categories of age, AFP, β-HCG, and LDH. A chi-square test/Fisher's exact test was run to check the association of outcome and recurrence of disease with demographic, laboratory, and clinical parameters. The Mann–Whitney test was employed to assess the median difference in AFP, β-HCG, and LDH values. The Kaplan–Meier method was utilized to estimate the OS and EFS rates. Univariate and multivariate analyses were conducted using Cox's proportional hazard model. The significant parameters identified in the univariate analyses were subjected to multivariate analysis, and their association with recurrence/outcome was expressed as hazard ratios (HRs) with a 95%-confidence interval. A significance level of < 0.05 was set to determine a significant association between recurrence/outcome and the factors under investigation.
Ethical Approval
This analysis was conducted under an ethical exemption granted by the institutional ethical review board with the reference IHHN_IRB_2022_07_020 on August 3, 2022. All procedures performed in the study involving human participants adhered to the ethical principles outlined by the institutional and/or national research committee. The study also complied with the guidelines set forth in the 1964 Helsinki Declaration and its subsequent amendments or equivalent ethical standards.
Results
This retrospective study included a cohort of 97 patients, with a median age of 4 years (1.9–10) at the time of diagnosis. The male-to-female ratio was approximately 1:1.7, with males having a median age of 2 years (1.5–3.5) and females having a median age of 8.4 years (2.5–11).
According to the histopathology, the majority were the yolk sac tumor (YST) (50.5%), followed by dysgerminoma (17.5%). The study included a total of 97 patients, with 33 (34%) classified as HR, 35 (36.1%) classified as IR, and 29 (29.9%) classified as LR. The disease was metastasized in 28 (29%) patients, 17 (60.7%) had pulmonary metastatic diseases while 11 (39.3%) had nonpulmonary metastatic diseases at the time of diagnosis. The surgical excision was done in 84 (86.6%) patients, in which 55 (65%) had residual disease treated with adjuvant first-line chemotherapy and 29 (35%) were LR with complete surgical excision. The tumor was nonresectable in 13 (13.4%) patients, and neoadjuvant chemotherapy was given.
The first-line chemotherapy (JEB) was given to 68 (70%) patients. The recurrence has occurred in 16 patients in which 6 (38%) were from the LR group (chemo-naive) patients, 1 (6%) patient from IR, and 9 (56%) were from the HR group. The IR and HR patients were offered the second-line chemotherapy (TIP).
Out of the total patient population, 90 (92.8%) were reported as alive, while 7 patients (7.2%) had unfortunately passed away; the nonsignificant association of recurrence and outcomes with demographic, clinical, and laboratory parameters are depicted in [Table 1].
|
Characteristics |
Recurrence |
p-Value |
Outcomes |
p-Value |
||
|---|---|---|---|---|---|---|
|
Yes (n = 16) |
No (n = 81) |
Alive (n = 90) |
Expired (n = 7) |
|||
|
Age (y) (n) (%) |
||||||
|
< 10> > 10 |
13 (81.3) 03 (18.8) |
56 (69.1) 25 (30.9) |
0.385[a] |
63 (70) 27 (30) |
06 (85.7) 01 (14.3) |
0.669[a] |
|
Sex (n) (%) |
||||||
|
Male Female |
04 (25) 12 (75) |
31 (38.3) 50 (61.7) |
0.312[b] |
32 (35.6) 58 (64.4) |
03 (42.9) 04 (57.1) |
1.000[a] |
|
Histology (n) (%) |
||||||
|
Mature teratoma |
01 (6.3) |
10 (12.3) |
0.685[a] |
11 (12.2) |
0 |
1.000[a] |
|
Immature components (teratoma) |
01 (6.3) |
04 (4.9) |
1.000[a] |
05 (5.6) |
0 |
1.000[a] |
|
Dysgerminoma |
02 (12.5) |
15 (18.5) |
0.730[a] |
16 (17.8) |
01 (14.3) |
1.000[a] |
|
Mixed GCT |
03 (18.8) |
09 (11.1) |
0.412[a] |
11 (12.2) |
01 (14.3) |
1.000[a] |
|
Yolk sac tumor |
09 (56.3) |
40 (49.4) |
0.616[b] |
44 (48.9) |
05 (71.4) |
0.436[a] |
|
Embryonal carcinoma |
0 |
03 (3.7) |
1.000[a] |
03 (3.3) |
0 |
1.000[a] |
|
Risk (n) (%) |
||||||
|
Low risk Intermediate risk High risk |
06 (37.5) 01 (6.3) 09 (56.3) |
23 (28.4) 34 (42) 24 (29.6) |
0.019[b] |
29 (32.2) 31 (34.4) 30 (33.3) |
0 04 (57.1) 03 (42.9) |
0.191[a] |
|
Metastatic disease (n) (%) |
||||||
|
Yes No |
05 (31.3) 11 (68.8) |
23 (28.4) 58 (71.6) |
0.772[a] |
27 (30) 63 (70) |
01 (14.3) 06 (85.7) |
0.669[a] |
|
AFP (ng/mL) (n) (%) |
||||||
|
< 10> > 10,000 Normal |
03 (18.8) 10 (62.5) 03 (18.8) |
28 (34.6) 30 (37) 23 (28.4) |
0.164[b] |
29 (32.2) 35 (38.9) 26 (28.9) |
02 (28.6) 05 (71.4) 0 |
0.169[a] |
|
HCG (IU/L) (n) (%) |
||||||
|
< 5> > 5,000 |
14 (87.5) 02 (12.5) |
73 (94.8) 04 (5.2) |
0.274[a] |
80 (92.9) 06 (7.1) |
07 (100) 0 |
1.000[a] |
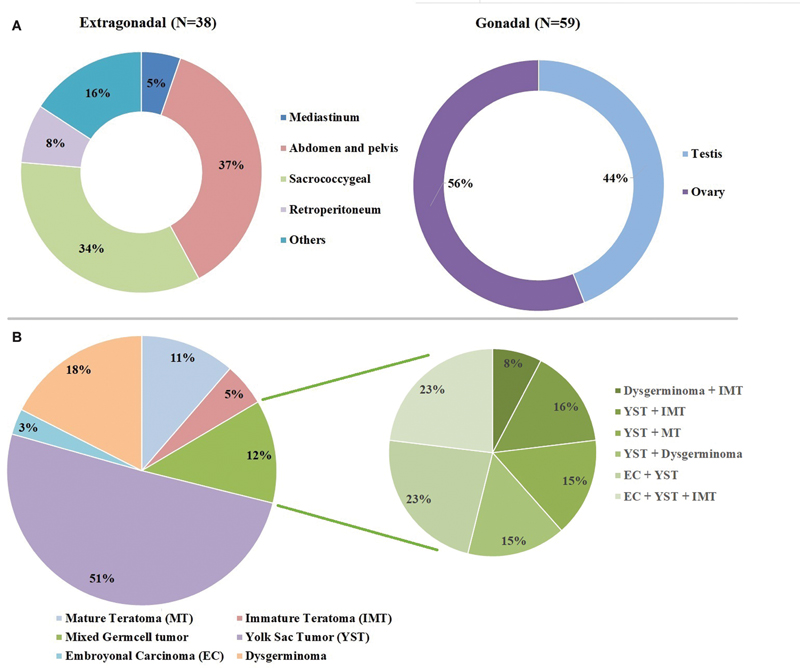
| Fig 1 : (A) Anatomical distribution of gonadal and extragonadal germ cell tumors. (B) Distribution according to histopathology.
In gonadal GCT, in testis, the most common was YST, 21 (80.8%), followed by mixed GCT (MGCT) 3 (11.5%) and each of one case of mature and immature teratoma (IMT). In ovarian tumor, dysgerminoma, 14 (42.4%), was the most common, followed by YST 8 (24.2%), MGCT 6 (18.2%), 1 (3%) MT, and two of each case of embryonal carcinoma (EC) and IMT.
Total 49 cases of YST were reported in our study, the AFP level was high ≥ 10,000 (ng/mL) in 34 (69.4%), < 10,000 (ng/mL) were 13 (26.5%), and 2 (4.1%) were in normal range.
The histopathological distribution is shown in [Fig. 1B], YST and dysgerminoma were commonly found in 49 and 17 patients, respectively. MT and IMT were in 11 and 5 patients, respectively, however, 3 were EC.
MGCT was found in 12 patients; we have reported 3 patients in each MGCT with EC + YST and IMT + EC + YST. Two patients in each MGCT with YST + dysgerminoma, YST + IMT, and YST + MT. One in each MGCT with IMT + dysgerminoma ([Fig. 1B]).
Survival Analysis
The study followed all patients for a median duration of 17.2 months (8.2–25.3). Seven patients died, 03 were related to progressive disease, 01 had secondary malignancy (brain tumor), 02 had bacterial and fungal infections, and 01patient had a recurrence of the disease.
Recurrence has occurred in 16 patients in which 10 (62.5%) were off treatment after first-line chemotherapy and 06 (37.5%) were LR chemo-naive patients. In these 6 patients, 1 had lymphovascular invasion and 2 patients had mixed histology, while 3 had dysgerminoma and 1 YST.
For the entire patient cohort (n = 97), the 5-year EFS rate was determined to be 83.5%, while the 5-year OS rate was calculated as 92.8% ([Fig. 2A]).
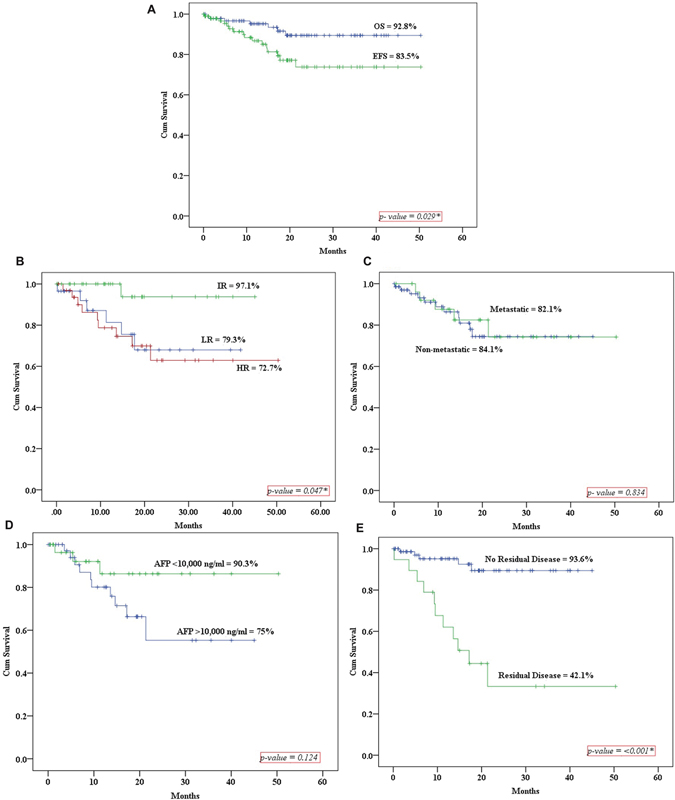
| Figure 2: (A) The 5-year event-free survival (EFS) and overall survival (OS). (B–E) The 5-year EFS according to the risk factors (IR = intermediate risk; LR = low-risk; HR = high-risk; AFP = α-fetoprotein.).
[Fig. 2B] shows the 5-year EFS rates among different risk groups (LR, IR, and HR), demonstrating a statistically significant difference. Owing to female predominance and primary nononcological surgeries outside the tertiary care center, we found lower EFS in the LR group as compared with the IR group.
Furthermore, [Fig. 2C] illustrates the EFS rates for children without metastases (84.1%) and those with metastases (82.1%), showing no statistically significant difference. In [Fig. 2D], patients with AFP < 10,000 ng/mL exhibited a higher EFS compared with those with elevated AFP ≥ 10,000 ng/mL, although the difference was not statistically significant. The EFS of patients after the first-line treatment with the small residual disease was 42.1% as compared with patients with complete remission, 93.6%, which was statistically significant ([Fig. 2E]). Through multivariable analysis, it was determined that the presence of residual disease emerged as the sole independent prognostic factor, with a HRs of 7.42. The residual disease was small in size and was not amenable for surgery without mutilation. We did not encounter condition like growing teratoma syndrome. Also, patients in the HR group and AFP level > 10,000 ng/mL were found to have 2.4 and 1.3 times higher HRs, respectively, which was statistically nonsignificant. There were 36% higher chances of patients with metastatic disease to have disease recurrence, but it was statistically nonsignificant ([Table 2]).
|
HRs |
95% CI |
p-Value |
|
|---|---|---|---|
|
High-risk group |
2.416 |
0.656–8.895 |
0.185 |
|
Metastatic disease |
0.361 |
0.098–1.331 |
0.126 |
|
AFP > 10,000 (ng/mL) |
1.370 |
0.424–4.422 |
0.598 |
|
Residual diseases |
7.426 |
2.455–22.46 |
< 0 href="#FN23791208-6" class="alt">a] |
References
- Cecchetto G. Gonadal germ cell tumors in children and adolescents. J Indian Assoc Pediatr Surg 2014; 19 (04) 189-194
- Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol 2018; 2018: 9059382
- Rescorla FJ. Pediatric germ cell tumors. Semin Pediatr Surg 2012; 21 (01) 51-60
- Schneider DT, Terenziani M, Cecchetto G. et al. Gonadal and extragonadal germ cell tumors, sex cord stromal and rare gonadal tumors. Chapter 39. In: Rare Tumors in Children and Adolescents. Germany: Springer Berlin Heidelberg; 2012: 327-402
- Parida L. Nonurological malignancies in children. J Indian Assoc Pediatr Surg 2014; 19 (01) 31-37
- Panteli C, Curry J, Kiely E. et al. Ovarian germ cell tumours: a 17-year study in a single unit. Eur J Pediatr Surg 2009; 19 (02) 96-100
- İncesoy-Özdemir S, Ertem U, Şahin G. et al. Clinical and epidemiological characteristics of children with germ cell tumors: a single center experience in a developing country. Turk J Pediatr 2017; 59 (04) 410-417
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997; 15 (02) 594-603
- Frazier AL, Hale JP, Rodriguez-Galindo C. et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 2015; 33 (02) 195-201
- Billmire DF, Cullen JW, Rescorla FJ. et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children's Oncology Group. J Clin Oncol 2014; 32 (05) 465-470
- Rescorla FJ, Ross JH, Billmire DF. et al. Surveillance after initial surgery for Stage I pediatric and adolescent boys with malignant testicular germ cell tumors: report from the Children's Oncology Group. J Pediatr Surg 2015; 50 (06) 1000-1003
- Goldman S, Bouffet E, Fisher PG. et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for non-germinomatous germ cell tumors: a Children's Oncology Group study. J Clin Oncol 2015; 33 (22) 2464-2471
- Fresneau B, Orbach D, Faure-Conter C. et al. Sex-cord stromal tumors in children and teenagers: results of the TGM-95 study. Pediatr Blood Cancer 2015; 62 (12) 2114-2119
- Islam Nasir IU, Ashraf MI, Ahmed N. et al. Clinical profile, treatment and survival outcomes of peadiatric germ cell tumours: a Pakistani perspective. J Pak Med Assoc 2016; 66 (10) S119-S121
- Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 2015; 135 (01) e136-e143
- Kumar H, Saju SV, Radhakrishnan V. et al. Analysis of extra-cranial germ cell tumors in male children: experience from a single centre in India. Pediatric Hematology Oncology Journal 2020; 5 (02) 37-42
- Marina N, London WB, Frazier AL. et al. Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol 2006; 24 (16) 2544-2548
- Lorch A, Bascoul-Mollevi C, Kramar A. et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol 2011; 29 (16) 2178-2184
-
Rescorla FJ. Pediatric germ cell tumors. Semin Surg Oncol 1999; 16 (02) 144-158
3.0.CO;2-M" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/(SICI)1098-2388(199903)16:2<144>3.0.CO;2-M" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">CrossrefPubMedSearch in Google Scholar
- PDQ Pediatric Treatment Editorial Board. Childhood Extracranial Germ Cell Tumors Treatment (PDQ®): Health Professional Version. 2023 Feb 10. In: PDQ Cancer Information Summaries [Internet]. Bethesda, MD: National Cancer Institute (US); 2002
- Wollner N, Ghavimi F, Wachtel A, Luks E, Exelby P, Woodruff J. Germ cell tumors in children: gonadal and extragonadal. Med Pediatr Oncol 1991; 19 (04) 228-239
- Depani S, Stoneham S, Krailo M, Xia C, Nicholson J. Results from the UK Children's Cancer and Leukaemia Group study of extracranial germ cell tumours in children and adolescents (GCIII). Eur J Cancer 2019; 118: 49-57
- Akyüz C, Varan A, Büyükpamukçu N, Kutluk T, Büyükpamukçu M. Malignant ovarian tumors in children: 22 years of experience at a single institution. J Pediatr Hematol Oncol 2000; 22 (05) 422-427
- Feltbower RG, Siller C, Woodward E. et al. Treatment and survival patterns for germ cell tumors among 13- to 24-year olds in Yorkshire, UK. Pediatr Blood Cancer 2011; 56 (02) 282-288
- Mann JR, Raafat F, Robinson K. et al. The United Kingdom Children's Cancer Study Group's second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000; 18 (22) 3809-3818
- Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 2015; 135 (01) e136-e143
- Bhuta R, Shah R, Gell JJ. et al. Children's Oncology Group's 2023 blueprint for research: Germ cell tumors. Pediatr Blood Cancer 2023; 70 (suppl 6, suppl 6): e30562
- Mason JB, Srivastava A, Lanzotti NJ. et al. Variations in germ cell tumor histology by age and implications for cancer-specific survival among pediatric and adult males: a population-based study. Urol Oncol 2024; 42 (09) 292.e17-292.e26
Address for correspondence
Publication History
Article published online:
24 February 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Second Malignant Neoplasms in Children and Adolescents Treated for Blood Malignancies and Solid Tumors: A Single-Center Experience of 15 YearsNikolaos Katzilakis, Indian Journal of Medical and Paediatric Oncology, 2018
- Mediastinal Germ Cell TumorsAlan Sandler, Semin Respir Crit Care Med, 1997
- Supratentorial and Infratentorial Approaches to Pineal Surgery: A Database AnalysisDavid M. Rosenberg, Journal of Neurological Surgery Part B: Skull Base
- Mediastinal Germ Cell TumorsAlan Sandler, Seminars in Respiratory and Critical Care Medicine, 1997
- Peculiarities of Yolk Sac Tumor in Head and Neck: A Case Report and Literature ReviewK Devaraja, Indian Journal of Medical and Paediatric Oncology, 2018
- Oncological Outcomes in Japanese Men Undergoing Orchiectomy for Stage I Testicular Germ Cell Tumor<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Brain Metastases from Malignant Germ Cell Tumors of the Testis: Our Experience of 5 Cases with a Review of the Literature<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- A Unique Case of Bilateral Synchronous Testicular Tumor with Concomitant Bilateral Diffuse Intratubular Germ Cell Neoplasia: Testis Sparing Surgery and Local Ra...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Testicular Metastasis from Ileal Adenocarcinoma: A Case Report and Review of Literature<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Plerixafor mobilization of peripheral blood hematopoietic progenitors to support further high-dose chemotherapy cycles in a patient with germ-cell tumor relapsi...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

| Fig 1 : (A) Anatomical distribution of gonadal and extragonadal germ cell tumors. (B) Distribution according to histopathology.

| Figure 2: (A) The 5-year event-free survival (EFS) and overall survival (OS). (B–E) The 5-year EFS according to the risk factors (IR = intermediate risk; LR = low-risk; HR = high-risk; AFP = α-fetoprotein.).
References
- Cecchetto G. Gonadal germ cell tumors in children and adolescents. J Indian Assoc Pediatr Surg 2014; 19 (04) 189-194
- Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol 2018; 2018: 9059382
- Rescorla FJ. Pediatric germ cell tumors. Semin Pediatr Surg 2012; 21 (01) 51-60
- Schneider DT, Terenziani M, Cecchetto G. et al. Gonadal and extragonadal germ cell tumors, sex cord stromal and rare gonadal tumors. Chapter 39. In: Rare Tumors in Children and Adolescents. Germany: Springer Berlin Heidelberg; 2012: 327-402
- Parida L. Nonurological malignancies in children. J Indian Assoc Pediatr Surg 2014; 19 (01) 31-37
- Panteli C, Curry J, Kiely E. et al. Ovarian germ cell tumours: a 17-year study in a single unit. Eur J Pediatr Surg 2009; 19 (02) 96-100
- İncesoy-Özdemir S, Ertem U, Şahin G. et al. Clinical and epidemiological characteristics of children with germ cell tumors: a single center experience in a developing country. Turk J Pediatr 2017; 59 (04) 410-417
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997; 15 (02) 594-603
- Frazier AL, Hale JP, Rodriguez-Galindo C. et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 2015; 33 (02) 195-201
- Billmire DF, Cullen JW, Rescorla FJ. et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children's Oncology Group. J Clin Oncol 2014; 32 (05) 465-470
- Rescorla FJ, Ross JH, Billmire DF. et al. Surveillance after initial surgery for Stage I pediatric and adolescent boys with malignant testicular germ cell tumors: report from the Children's Oncology Group. J Pediatr Surg 2015; 50 (06) 1000-1003
- Goldman S, Bouffet E, Fisher PG. et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for non-germinomatous germ cell tumors: a Children's Oncology Group study. J Clin Oncol 2015; 33 (22) 2464-2471
- Fresneau B, Orbach D, Faure-Conter C. et al. Sex-cord stromal tumors in children and teenagers: results of the TGM-95 study. Pediatr Blood Cancer 2015; 62 (12) 2114-2119
- Islam Nasir IU, Ashraf MI, Ahmed N. et al. Clinical profile, treatment and survival outcomes of peadiatric germ cell tumours: a Pakistani perspective. J Pak Med Assoc 2016; 66 (10) S119-S121
- Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 2015; 135 (01) e136-e143
- Kumar H, Saju SV, Radhakrishnan V. et al. Analysis of extra-cranial germ cell tumors in male children: experience from a single centre in India. Pediatric Hematology Oncology Journal 2020; 5 (02) 37-42
- Marina N, London WB, Frazier AL. et al. Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol 2006; 24 (16) 2544-2548
- Lorch A, Bascoul-Mollevi C, Kramar A. et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol 2011; 29 (16) 2178-2184
-
Rescorla FJ. Pediatric germ cell tumors. Semin Surg Oncol 1999; 16 (02) 144-158
3.0.CO;2-M" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/(SICI)1098-2388(199903)16:2<144>3.0.CO;2-M" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">CrossrefPubMedSearch in Google Scholar
- PDQ Pediatric Treatment Editorial Board. Childhood Extracranial Germ Cell Tumors Treatment (PDQ®): Health Professional Version. 2023 Feb 10. In: PDQ Cancer Information Summaries [Internet]. Bethesda, MD: National Cancer Institute (US); 2002
- Wollner N, Ghavimi F, Wachtel A, Luks E, Exelby P, Woodruff J. Germ cell tumors in children: gonadal and extragonadal. Med Pediatr Oncol 1991; 19 (04) 228-239
- Depani S, Stoneham S, Krailo M, Xia C, Nicholson J. Results from the UK Children's Cancer and Leukaemia Group study of extracranial germ cell tumours in children and adolescents (GCIII). Eur J Cancer 2019; 118: 49-57
- Akyüz C, Varan A, Büyükpamukçu N, Kutluk T, Büyükpamukçu M. Malignant ovarian tumors in children: 22 years of experience at a single institution. J Pediatr Hematol Oncol 2000; 22 (05) 422-427
- Feltbower RG, Siller C, Woodward E. et al. Treatment and survival patterns for germ cell tumors among 13- to 24-year olds in Yorkshire, UK. Pediatr Blood Cancer 2011; 56 (02) 282-288
- Mann JR, Raafat F, Robinson K. et al. The United Kingdom Children's Cancer Study Group's second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000; 18 (22) 3809-3818
- Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 2015; 135 (01) e136-e143
- Bhuta R, Shah R, Gell JJ. et al. Children's Oncology Group's 2023 blueprint for research: Germ cell tumors. Pediatr Blood Cancer 2023; 70 (suppl 6, suppl 6): e30562
- Mason JB, Srivastava A, Lanzotti NJ. et al. Variations in germ cell tumor histology by age and implications for cancer-specific survival among pediatric and adult males: a population-based study. Urol Oncol 2024; 42 (09) 292.e17-292.e26


 PDF
PDF  Views
Views  Share
Share

