Gestational trophoblastic neoplasia, management as per risk stratification in a developing country
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 28-31
DOI: DOI: 10.4103/0971-5851.177012
Abstract
Aims: The purpose of this analysis was to address the outcome of GTN from a tertiary care centre of India. Materials and Methods: We undertook a retrospective and prospective review of GTN cases treated at our centre from 2006 to 2014. Patients of GTN were assigned to low-risk or high-risk categories as per the FIGO scoring system. The low-risk group was treated with combination of actinomycin-D and methotrexate (MTX) and the high-risk group received the EMA/CO regimen. Salvage therapy was EP/TP. Treatment was continued for 3 cycles after normalization of β-hCG level, after which the patients were kept on follow-up. Results: In total, 52 GTN patients were treated at our institution during this period; 21 were low-risk and 31 were in the high-risk category. The lung was the most common site of metastasis. All low risk patients achieved complete remission. Among high risk patients one patient died while receiving first cycle chemotherapy, one patient relapsed and 29 patients achieved complete remission. The single relapsed patient also achieved remission with 2nd line chemotherapy. Conclusion: 1. Two drug combination of Actinomycin-D and Methotrexate is a better alternative to single drug chemotherapy especially in developing countries were proper risk stratification is not always possible. 2. Patients with high disease burden should initially be treated with low dose chemotherapy to avoid life threatening visceral haemorrhage.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
The purpose of this analysis was to address the outcome of GTN from a tertiary care centre of India.
Materials and Methods:
We undertook a retrospective and prospective review of GTN cases treated at our centre from 2006 to 2014. Patients of GTN were assigned to low-risk or high-risk categories as per the FIGO scoring system. The low-risk group was treated with combination of actinomycin-D and methotrexate (MTX) and the high-risk group received the EMA/CO regimen. Salvage therapy was EP/TP. Treatment was continued for 3 cycles after normalization of β-hCG level, after which the patients were kept on follow-up.
Results:
In total, 52 GTN patients were treated at our institution during this period; 21 were low-risk and 31 were in the high-risk category. The lung was the most common site of metastasis. All low risk patients achieved complete remission. Among high risk patients one patient died while receiving first cycle chemotherapy, one patient relapsed and 29 patients achieved complete remission. The single relapsed patient also achieved remission with 2nd line chemotherapy.
Conclusion:
1. Two drug combination of Actinomycin-D and Methotrexate is a better alternative to single drug chemotherapy especially in developing countries were proper risk stratification is not always possible. 2. Patients with high disease burden should initially be treated with low dose chemotherapy to avoid life threatening visceral haemorrhage.
INTRODUCTION
Gestational trophoblastic disease (GTD) can be benign or malignant. Histologically, it is classified into hydatidiform mole invasive mole (chorioadenoma destruens), choriocarcinoma, and placental site trophoblastic tumor. Those that invade locally or metastasize are collectively known as gestational trophoblastic neoplasia (GTN). Hydatidiform mole is the most common form of GTN. While invasive mole and choriocarcinoma are malignant, a hydatidiform mole can behave in a malignant or benign fashion. We report the clinical profile, management, and outcome of GTN patients treated at our center. The study was conducted over a period of 8 years.
MATERIALS AND METHODS
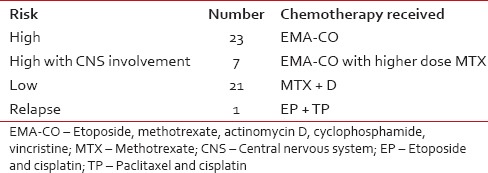
The data were extracted by retrieving all case records of GTN patients registered during the study period. The abstracted information included history and examination findings; chest X-ray, pelvic ultrasound, computed tomography (if carried out), magnetic resonance imaging (MRI) brain, cerebrospinal fluid (CSF) (as and when required) and serum human chorionic gonadotropin (β-hCG), and histopathological evaluation of biopsy or curettage specimen, if available. Using this information, patients were categorized as low-risk or high-risk according to the International Federation of Gynecology and Obstetrics (FIGO) scoring system. Patients with a score of ≤6 were treated with methotrexate (MTX) and actinomycin D combination. The MTX was given at a dose of 300 mg/m2 over 4 h with calcium leucovorin rescue and actinomycin D at a dose of 0.5 mg on day 1. The cycles were repeated 2 weekly. Serum β-hCG levels were measured once every week, including before the start of every chemotherapy cycle; any patient who had two static or one increasing value on treatment was defined as having drug-resistant disease. Patients with a score ≥7 were classified as high-risk and started on the etoposide, methotrexate, actinomycin D, and cyclophosphamide, vincristine (EMA/CO) regimen. Patients who relapsed were subsequently treated with EP/TP regimen. The patients with CNS disease received EMA-CO with a higher dose of MTX (1 g/m2) [Table 1]. Treatment was continued for 3 cycles after normalization of serum β-hCG in high-risk group and for 2 cycles in low-risk group. After the last chemotherapy cycle, the patients were kept on regular follow-up using regular β-hCG monitoring as per standard guidelines.[1,2] Specifically, β-hCG level was measured 6-8 weeks after the end of any future pregnancy to exclude disease recurrence.[3,4] Patients were also advised standard contraceptive measures.[5] The response to therapy was defined as follows: Complete response as β-hCG values in the normal range for 3 consecutive weeks, a partial response as more than 50% decline in β-hCG levels compared with baseline, no response as < 50% decline over baseline values, and progressive disease as an increase of at least 25% in the size of any measurable lesion or appearance of any new lesion with increasing β-hCG levels. Recurrence was defined as elevation of β-hCG level after more than three normal values in the absence of a confirmed pregnancy.[6] All the female patients irrespective of age with diagnosis of GTN were taken for study.
Table 1
Chemotherapy protocols that patient received as per risk stratification
RESULTS
Of 52 patients diagnosed with GTN, diagnosis was based on histopathological evidence in 6 and elevated serum β-hCG and history consistent with GTN in 46 patients. The most common presenting feature was bleeding per vagina which was present in 48 patients. The passage of grape-like vesicles was present in 8 patients. Other features at presentation included hemoptysis (6 patients), pain abdomen (6 patients), and excessive vomiting was noted in 3 patients.
On examination, the most common finding was pallor which was noted in 28 patients, in 1 patient there was mild abdominal tenderness, the examination was normal in rest of the patients. Of 52 patients, 31 were in high-risk category and 21 were in low-risk category. The median age of our patients was 32 years (20-46 years). Only 3 patients were more than 40 years of age and all of them were in high-risk group. The FIGO Stage 1 comprised the largest staging group among our patients (26 patients) and FIGO 4, the smallest group (4 patients). All stage 4 patients belonged to high-risk group only. The antecedent pregnancy was term in 5, abortion in 17, and molar in 30. Interval from antecedent pregnancy to initiation of chemotherapy was < 4 months in 19 patients, between 4 and 7 months in 20 patients, between 7 and 12 months in 8 patients, and more than 12 months in 5 patients. The average preevacuation serum β-hCG was 177,000 IU/L, in high-risk patients, average preevacuation serum β-hCG was 270,000 IU/L (19,000-1410,000 IU/L) while in low-risk patients, it was 80,000 IU/L (4500-462,417 IU/L). The average overall postevacuation serum β-hCG was 92,000 IU/L (2800-726,151 IU/L), in high-risk patients, average postevacuation serum β-hCG was 132,000 IU/L (3620-726,151 IU/L), while in low-risk patients, it was 49,500 IU/L (2800-509,991 IU/L). The lung metastasis was found in 15 patients and CNS was involved in 7 patients, 1 patient was having MRI documented brain metastasis, and other 6 had high CSF/serum β-hCG. Among high-risk patients, lung metastases were present in 14 patients and CNS involvement was present in 7 patients. Among low-risk patients, only 1 patient had lung metastasis. The size of largest tumor was < 3 cm in 20 patients, 3-5 cm in 25 patients, and > 5 cm in 7 patients. The number of metastases was 1-4 in 4 patients of high-risk group and more than 4 in 1 patient of high-risk group. In low-risk group, the number of metastases was 0 in all patients (as per ESMO guidelines the lung metastases were counted on chest X-ray only and not on chest contrast-enhanced computed tomography).
Only 2 patients had a history of prior failed chemotherapy and both of them were in high-risk group. The number of chemotherapy cycles received on average for normalization of serum β-hCG levels was 4.82 (2-12). In high-risk group, on average, patients received 5.1 (3-8), while in low-risk group, patients received 4.46 cycles (2-12) for normalization of serum β-hCG levels.
Total number of chemotherapy cycles received on average was 7.08 (4-14). In high-risk patients, average number of cycles received was 7.6 (4-11), while in low-risk patients, it was 6.13 (4-14).
All low-risk patients attained remission with combination of actinomycin D and MTX (2 years survival: 100%). Among high-risk patients, 1 patient relapsed and 1 patient died while receiving the first cycle of chemotherapy (2 years survival: 97%).
Among low-risk patients, 5 patients had normal deliveries while among high-risk patients, 4 had normal deliveries during the follow-up period.
Single patient relapsed in high-risk group and there was no relapse in low-risk group. The lone relapsed patient was treated with 2 weekly cycles of cisplatin (60 mg/m2)and paclitaxel (135 mg/m2) alternately with paclitaxel (135 mg/m2) and etoposide (150 mg/m2).
DISCUSSION
There is no consensus on the best chemotherapy regimen for initial management of low-risk GTN, and first-line regimens vary by geography and institutional preference. Most regimens have not been compared head-to-head, and the level of evidence for efficacy is often limited.
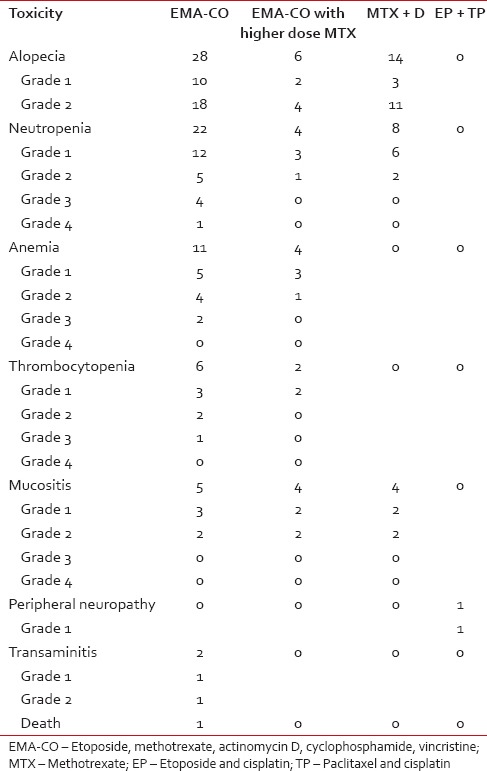
As gestational trophoblastic neoplasia is a highly curable disease, the aim of treatment should be to minimize the drug toxicity, but not at the cost of treatment efficacy. In our patients, these goals were demonstrably achieved as shown in Table 2. The combination chemotherapy has definite advantages over single agent chemotherapy in low-risk patients. All the low-risk patients who were treated with methotrexate and actinomycin D combination (21 patients) achieved remission, and there was no relapse in this subset of patients. In other study from India, 92.9% of postmolar GTN attained complete remission with MTX.[7] Other studies from developed countries have reported lower rates of remission (72%) with methotrexate and higher requirement of second-line salvage regimens.[8] Although it is difficult to draw definitive conclusions, it is possible that there may not be proper risk stratification in developing countries and patients with risk scores of 5 and 6 may be relapsing on single agent chemotherapy. The two drug regimens received by our patients have prevented relapses, but at the same time, it has caused minimal toxicity [Table 3]. Only 6 patients developed neutropenia and 2 patients developed low-grade mucositis. It is pertinent to mention here that no patient needed growth factor support, and there was no delay in chemotherapy because of neutropenia. The patients in our chemotherapy protocol received 300 mg/m2 of MTX over 4 h with 4 doses of calcium leucovorin and single dose actinomycin D 0.5 mg and were discharged on the same day. The cycle was repeated 2 weekly. They said chemotherapy protocol could be a good option for low-risk patients, especially in developing countries.
Table 2
Summarizes the demographic, baseline characteristics, and treatment outcome of all patients
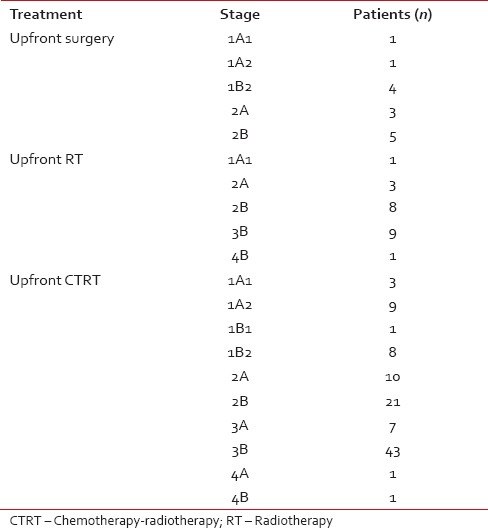
Table 3
The toxicity of chemotherapy
Our treatment outcomes are comparable with national and international data in low-risk disease.[8,9,10,11]
Among the high-risk patients in our study, a large majority (91.6%) achieved complete remission with the first line use of the EMA/CO regimen. The long-term survival was 96%, which is in the same range as most other centers that treat high-risk disease.[12]
The one high-risk patient who relapsed on EMA-CO attained remission with second-line salvage chemotherapy.
Although the number of patients treated at our institute was small, the survival was at par with national and international data.[11,13,14,15,16] The single patient who died was having high disease burden and died while receiving the first cycle of chemotherapy likely due to pulmonary hemorrhage. These deaths in future could be avoided by starting with low dose chemotherapy for 2-3 cycles and then changing to usual EMA-CO chemotherapy in patients with high disease load.
CONCLUSION
- Two-drug combination of actinomycin D and MTX is a better alternative to single drug chemotherapy, especially in developing countries where proper risk stratification is not always possible.
- EMA-CO is appropriate for use in experienced centers in developing countries in appropriately risk-stratified patients.
- Patients with high disease burden should initially be treated with low-dose chemotherapy to avoid life-threatening visceral hemorrhage.
- The greater number of patients with high-risk disease in our study may be due to more time taken by patients to seek medical advice after an antecedent event.
- The relatively low rate of documented fertility in our data is probably a reflection of the completion of family at a young age in our community and effective adherence to contraceptive advice. It is also possible that there has been less than perfect long-term follow-up of these patients with respect to their reproductive outcomes.
- It may be concluded that 1 g/m2 MTX is a good choice for patients with CNS disease.
- In summary, our data show that very high rates of remission and survival are possible in both low- and high-risk GTN patients in developing countries.
Such patients should preferably be referred to experienced centers that have capabilities for appropriate risk stratification and consequent treatment, including good supportive care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

