Harmful effects of nicotine
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(01): 24-31
DOI: DOI: 10.4103/0971-5851.151771
Abstract
With the advent of nicotine replacement therapy, the consumption of the nicotine is on the rise. Nicotine is considered to be a safer alternative of tobacco. The IARC monograph has not included nicotine as a carcinogen. However there are various studies which show otherwise. We undertook this review to specifically evaluate the effects of nicotine on the various organ systems. A computer aided search of the Medline and PubMed database was done using a combination of the keywords. All the animal and human studies investigating only the role of nicotine were included. Nicotine poses several health hazards. There is an increased risk of cardiovascular, respiratory, gastrointestinal disorders. There is decreased immune response and it also poses ill impacts on the reproductive health. It affects the cell proliferation, oxidative stress, apoptosis, DNA mutation by various mechanisms which leads to cancer. It also affects the tumor proliferation and metastasis and causes resistance to chemo and radio therapeutic agents. The use of nicotine needs regulation. The sale of nicotine should be under supervision of trained medical personnel.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
With the advent of nicotine replacement therapy, the consumption of the nicotine is on the rise. Nicotine is considered to be a safer alternative of tobacco. The IARC monograph has not included nicotine as a carcinogen. However there are various studies which show otherwise. We undertook this review to specifically evaluate the effects of nicotine on the various organ systems. A computer aided search of the Medline and PubMed database was done using a combination of the keywords. All the animal and human studies investigating only the role of nicotine were included. Nicotine poses several health hazards. There is an increased risk of cardiovascular, respiratory, gastrointestinal disorders. There is decreased immune response and it also poses ill impacts on the reproductive health. It affects the cell proliferation, oxidative stress, apoptosis, DNA mutation by various mechanisms which leads to cancer. It also affects the tumor proliferation and metastasis and causes resistance to chemo and radio therapeutic agents. The use of nicotine needs regulation. The sale of nicotine should be under supervision of trained medical personnel.
INTRODUCTION
Tobacco is the leading cause of preventable cancers. WHO estimated around 1.27 billion tobacco users world-wide. Tobacco consumption alone accounts for nearly 5.4 million deaths per year and one billion people may die in this century if global tobacco consumption remained at the current levels.[1] An international treaty spearheaded by WHO in 2003 and signed by 170 countries, aims to encourage governments to reduce the production, sales, distribution advertisement and promotion of tobacco products. Despite strong opposition from the Industry, the treaty has been making steady progress in achieving its goal of comprehensive tobacco control around the world.[2] As tobacco consumption is being curbed, there is a growing demand for cessation. Pharmacological treatment of nicotine addiction remains an active area of research. There are many nicotine preparations (nicotine gums, patches, e cigarettes and inhalational agents) that are freely available in most parts of the world. These products are being heavily promoted and marketed as magical remedies. Nicotine gums are available in 2 mg and 4 mg preparation that deliver around 1 mg and 3 mg nicotine to the blood stream respectively. E-cigarette, a sophisticated nicotine delivery device, delivers nicotine in a vapor form and it closely mimics the act of smoking. Currently, these products constitute approximately 1% of total nicotine consumption and are showing an increasing trend in most countries.[3]
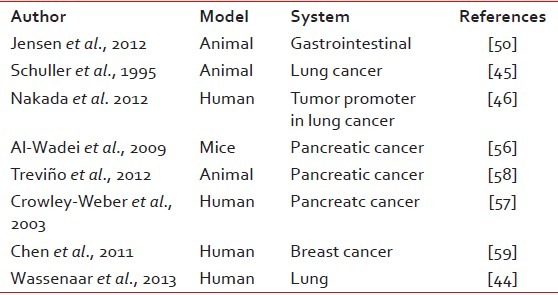
Nicotine is well known to have serious systemic side effects in addition to being highly addictive. It adversely affects the heart, reproductive system, lung, kidney etc. Many studies have consistently demonstrated its carcinogenic potential. [Table 1] The only other known use of nicotine has been as an insecticide since 17th century.[4] After World War II, its use has declined owing to the availability of cheaper, more potent pesticides that are less harmful to mammals. The environment Protection Agency of United States has banned use of nicotine as a pesticide from 1st January 2014.[4] India, one of the largest producer and exporter of nicotine sulphate, has progressively banned its use as agricultural pesticide.[5] We undertook this review to evaluate the systemic adverse effects of nicotine.
Table 1
Studies showing nicotine as a carcinogen
MATERIALS AND METHODS
A computer aided search of the Medline and PubMed databases was done using different combination of the keywords “nicotine,” “chemical composition,” “history,” “metabolism,” “addiction,” “cancer,” “toxic,” “endocrine system,” “cardiovascular system,” “respiratory system,” “lung carcinogenesis, “gastrointestinal system,” “immune system,” “ocular,” “cataract,” “central nervous system,” “renal system,” “reproductive system,” “menstrual cycle,” “oocytes,” “foetus,”. Initial search buildup was done using “Nicotine/adverse effects” [Mesh], which showed 3436 articles. Articles were analyzed and 90 relevant articles were included in the review. All the animal and human studies that investigated the role of nicotine on organ systems were analyzed. Studies that evaluated tobacco use and smoking were excluded. All possible physiological effects were considered for this review. We did not exclude studies that reported beneficial effects of nicotine. The objective was to look at the effects of nicotine without confounding effects of other toxins and carcinogens present in tobacco or tobacco smoke.
CHEMICAL PROPERTIES AND METABOLISM
Nicotine was first extracted from tobacco by German physicians Wilhelm Heinrich Posselt and Karl Ludwig Reimann. Nicotine, a strong alkaloid, in its pure form is a clear liquid with a characteristic odour. It turns brown on exposure to air. It is water soluble and separates preferentially from organic solvents. It is an amine composed of pyridine and pyrrolidine rings.
Nicotine is a dibasic compound and the availability and absorption in human body depends upon the pH of the solution.[7] The absorption can occur through oral mucosa, lungs, skin or gut.[6] The increase in pH of a solution causes an increase in concentrations of uncharged lipophilic nicotine, in this form it can actively pass through all biological membranes.[7] The addition of slaked lime and catechu to tobacco increases the absorption of nicotine from the oral cavity.
Nicotine once ingested, is absorbed and metabolized by the liver. The metabolic process can be categorized into two phases. In phase I there is microsomal oxidation of the nicotine via multiple pathways.[8] This leads to formation of various metabolites like cotinine and nornicotine, demethyl cotinine, trans-3-hydroxy-cotinine and d-(3-pyridyl)-g-methylaminobutyric acid.[9,10] Thereafter in phase II there is N’-and O’-glucuronidation of the metabolites and excretion via urine, feces, bile, saliva, sweat etc.[11,12]5-10% of elimination is by renal excretion of unchanged nicotine, however there is reabsorption from the bladder when the urinary pH is high.[14] There is evidence that nitrosation of nicotine in vivo could lead to formation of N-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).[13] which are known to be highly carcinogenic. Inflammation in the oral cavity increases risk of endogenous nitrosation.
MECHANISM OF ACTION
Nicotine acts via 3 major mechanisms, producing physiological and pathological effects on a variety of organ systems.[15,16]
- Ganglionic transmission.
- Nicotinic acetylcholine receptors (nAChRs) on chromaffin cells via catecholamines.
- Central nervous system (CNS) stimulation of nAChRs.
Brain imaging studies demonstrate that nicotine acutely increases activity in the prefrontal cortex and visual systems. There is release of a variety of neurotransmitters important in drug-induced reward. Nicotine also causes an increased oxidative stress and neuronal apoptosis, DNA damage, reactive oxygen species and lipid peroxide increase. nAChRs were originally thought to be limited to neuronal cells, however, studies have identified functional nAChRs in tissues outside the nervous system. Actions on nicotinic receptors produce a wide variety of acute and long-term effects on organ systems, cell multiplication and apoptosis, throughout the body.
IMMEDIATE EFFECTS AND TOXICITY
Nicotine on direct application in humans causes irritation and burning sensation in the mouth and throat, increased salivation, nausea, abdominal pain, vomiting and diarrhea.[17] Gastrointestinal effects are less severe but can occur even after cutaneous and respiratory exposure.[18] Predominant immediate effects as seen in animal studies and in humans consist of increase in pulse rate and blood pressure. Nicotine also causes an increase in plasma free fatty acids, hyperglycemia, and an increase in the level of catecholamines in the blood.[19,20] There is reduced coronary blood flow but an increased skeletal muscle blood flow.[20,22] The increased rate of respiration causes hypothermia, a hypercoagulable state, decreases skin temperature, and increases the blood viscosity.
Nicotine is one of the most toxic of all poisons and has a rapid onset of action. Apart from local actions, the target organs are the peripheral and central nervous systems. In severe poisoning, there are tremors, prostration, cyanosis, dypnoea, convulsion, progression to collapse and coma. Even death may occur from paralysis of respiratory muscles and/or central respiratory failure with a LD50 in adults of around 30-60 mg of nicotine. In children the LD50 is around 10 mg.[23]
GREEN TOBACCO SICKNESS
This is an acute form of nicotine toxicity that is known to occur due to handling of green tobacco leaves, with symptoms lasting from 12 to 24 h. The acute symptoms include headache, nausea, vomiting, giddiness, loss of appetite, fatigue and tachyarrythmias.[24] No significant mortality has been reported due to green tobacco sickness (GTS) but it significantly affects the health of workers in the tobacco industry.[25]
NICOTINE ADDICTION
Nicotine is one of the most addicting agent. The US surgeon general (2010) has concluded nicotine to be as addictive as cocaine or heroin. Nicotine interacts with the nicotinic acetyl choline receptors and stimulates the dopaminergic transmission.[26] This in turn stimulates the reward centre and is responsible for the mood elevation and apparent improvement in cognitive function.[27] With chronic stimulation by nicotine the GABAergic neurons are desensitized and thus lose their inhibitory effect on dopamine.[28] This in turn reinforces the addiction by inducing craving. This effect has been shown to affect the CYP2A6 gene and leads to heritable dependence to nicotine. Studies have shown the nicotine dependence to be transmitted maternally and grand maternally by epigenetic mechanism.[29]
EFFECTS ON METABOLISM
Nicotine causes catecholamine release and stimulates the autonomic system. There is increased glycogen synthesis due to α-adrenoceptor stimulation. This leads to reduction in the fasting blood glucose levels. It also causes lipolysis thus decreasing body weight. Nicotine affects insulin resistance and predisposes to metabolic syndrome. In an animal study prenatal exposure was toxic to pancreatic β-cell and leads to decreased B cell population, thus increasing the risk of diabetes.[30,31]
NICOTINE AND CANCER
The stimulation of nAChRs by nicotine has biologic effects on cells important for initiation and progression of cancer.[26] It activates signal transduction pathways directly through receptor-mediated events, allowing the survival of damaged epithelial cells. In addition, nicotine is a precursor of tobacco specific nitrosamines (TSNAs), through nitrosation in the oral cavity.[32,33] It is shown that nitrosation of nicotine could lead to formation of NNN and NNK. This effect of nicotine may be important because of its high concentration in tobacco and nicotine replacement products.[13] NNN and NNK are strongly carcinogenic.[34]
Nicotine forms arachidonic acid metabolites which cause increased cell division. Binding to Bcl-2 and action on vascular endothelial growth factor and cyclooxygenase-2 (COX-2) causes increased cancer proliferation and survival.[35,36] Promotion of tumor angiogenesis accelerates tumor growth which is mediated by β-adrenergic activation and stimulation of nAChRs.[35,37,28,39] Nicotine also suppresses apoptosis by phosphorylation mediated extracellular signal regulated kinases of Bcl-2.[40,41] Recent studies show that nicotine, activates nuclear factor kappa B (NF-kB)-dependent survival of cancer cell and proliferation.[42]
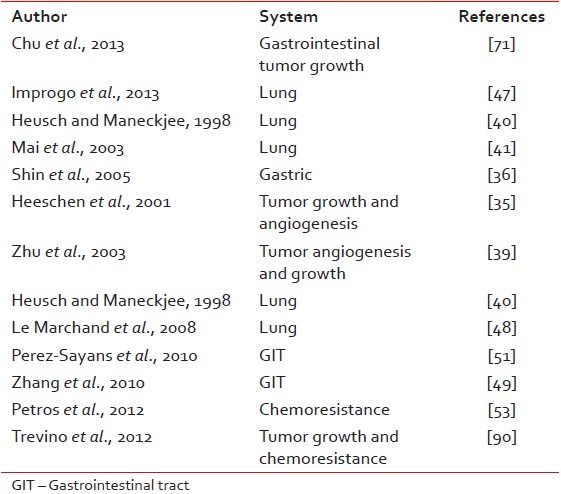
In normal cells, nicotine can stimulate properties consistent with cell transformation and the early stages of cancer formation, such as increased cell proliferation, decreased cellular dependence on the extracellular matrix for survival, and decreased contact inhibition. Thus, the induced activation of nAChRs in lung and other tissues by nicotine can promote carcinogenesis by causing DNA mutations[26] Through its tumor promoter effects, it acts synergistically with other carcinogens from automobile exhausts or wood burning and potentially shorten the induction period of cancers[43] [Table 2].
Table 2
Studies showing the role of nicotine as tumor promoter
LUNG CARCINOGENESIS
A study relates lung carcinogenesis by nicotine due to genetic variation in CYP2B6.[44] Its simultaneous exposure with hyperoxia has been found to induce cancer in hamsters.[45] Cotinine has been found to promote lung tumorigenesis by inhibiting anti-apoptotic pathway.[46] Nuclear translocation of ARB1 gene by nicotine has found in proliferation and progression of nonsmall-cell lung cancer. Several Studies have shown that nicotine has significant role in tumor progression and metastasis via CXCR4 and increased angiogenesis.[36,47] Carriers of the lung-cancer-susceptibility loci in their DNA extract more nicotine. Smokers carrying the gene CHRNA3 and CHRNA5 were found to extract more nicotine and cells were thus exposed to a higher internal dose of carcinogenic nicotine-derived nitrosamines.[48] Additionally modulation of the mitochondrial signaling pathway leads to resistance to the chemotherapeutic agents.[49]
GASTRO INTESTINAL CARCINOGENESIS
The carcinogenic role may be mediated by the MAPK/COX-2 pathways, α-7 nAchR and β-adrenergic receptor expression, and mi RNAs α-BTX anatagonist.[50] Nicotine forms adducts with liver DNA which enhances its mutagenic potential.[49,51,52] activation of cell-surface receptors by nicotine stimulates downstream kinases that can mediate resistance to chemotherapy. It has been shown by the finding that smokers who continue to smoke during chemotherapy have a worse prognosis. Moreover they also have increased toxicity and lower efficacy of chemo therapeutic drugs.[53] Nicotine affects the periostin gene, α-7-nAChR and e-cadherin suppression which explains the mechanism of gastric cancer growth, invasion and metastasis.[54,55] Nicotine negatively impacts tumor biology by promoting angiogenesis, tumor invasion and increased risk of metastasis.[53]
PANCREATIC CANCER
Nicotine has been found to induce pancreatic adenocarcinoma in mice model, by stimulating the stress neurotransmitters.[56,57] In another study nicotine promoted the growth of nonsmall cell lung cancer and pancreatic cancer in a receptor dependent fashion. It also increased tumor metastasis, and resistance to gemcitabine induced apoptosis, causing chemoresistance.[58] The MUC-4 upregulation, NF-kB and GRP78 activation and Id1 expression by Src dependent manner are the probable mechanism leading to tumor growth, metastasis and chemotherapeutic drug resistance.[57,58]
BREAST CANCER
Nicotine causes α9-nAChR-mediated cyclin D3 overexpression which might cause transformation of normal breast epithelial cells and induce cancer. Nicotine and cotinine has been found to be present in the breast fluid of lactating women.[59] Several studies have found that α9-nAChR mediated mechanism leads to increased tumor growth, metastasis and tumor cells resistant to chemotherapeutic drugs in breast cancer.[59,60]
CARDIOVASCULAR SYSTEM
The acute hemodynamic effects of cigarette smoking or smokeless tobacco are mediated primarily by the sympathomimetic action. The intensity of its hemodynamic effect is greater with rapid nicotine delivery.[61] Nicotine causes catecholamine release both locally and systemically leading to an increase in heart rate, blood pressure and cardiac contractility. It reduces blood flow in cutaneous and coronary vessels; and increases blood flow in the skeletal muscles. Due to restricted myocardial oxygen delivery there is reduced cardiac work. In a study, chewing a low dose (4 mg) of nicotine gum by healthy nonsmokers blunted the increase in coronary blood flow that occurs with increased heart rate produced by cardiac pacing.[21] Thus, persistent stimulation by nicotine can contribute to Coronary Vascular Disease by producing acute myocardial ischemia. In the presence of coronary disease, myocardial dysfunction can be worsened. In a placebo-controlled experiment that produced transient ischemia in anesthetized dogs myocardial dysfunction was produced at doses, that did not alter heart rate, blood pressure, or blood flow or myocyte necrosis.[62]
Nicotine alters the structural and functional characteristics of vascular smooth muscle and endothelial cells.[63] It enhances release of the basic fibroblast growth factor and inhibits production of transforming growth factor-β1.[64] These effects lead to increased DNA synthesis, mitogenic activity, endothelial proliferation and increases atherosclerotic plaque formation.[65] Neovascularization stimulated by nicotine can help progression of atherosclerotic plaques.[66] These effects lead to myointimal thickening and atherogenic and ischemic changes, increasing the incidence of hypertension and cardiovascular disorders. A study on dogs demonstrated the deleterious effects of nicotine on the heart.[67]
Nicotinic acetylcholine receptor's actions on vascular smooth muscle proliferation and plaque neovascularization increases the risk of peripheral arterial disorders. In a murine model of hind limb ischemia, short-term exposure to nicotine paradoxically increased capillary density and improved regional blood flow in the ischemic hind limb.[35] However, long-term exposure to nicotine for 16 weeks (about one-third of the life span of a mouse) before induction of ischemia obliterated angiogenic response to nicotine.[68]
RESPIRATORY SYSTEM
The effects of nicotine on respiratory system are twofold. One, directly by a local exposure of lungs to nicotine through smoking or inhaled nicotine, and second via a central nervous system mechanism. Nicotine plays a role in the development of emphysema in smokers, by decreasing elastin in the lung parenchyma and increasing the alveolar volume. Nicotine stimulates vagal reflex and parasympathetic ganglia and causes an increased airway resistance by causing bronchoconstriction.[69] Nicotine alters respiration through its effects on the CNS. The simultaneous effect of bronchoconstriction and apnea increases the tracheal tension and causes several respiratory disorders. In a study microinjection of nicotine were administered to the prebotzinger complex and adjacent nuclei in the brain. The firing pattern of the brain signals and breathing pattern were monitored. There was an increased frequency of bursts and decreased amplitude and a shallow and rapid rhythm of respiration.[70]
GASTROINTESTINAL SYSTEM
Nicotine use has been associated with Gastro Esophageal Reflux Disorder (GERD) and peptic ulcer disease (PUD).[36,71] This effect is mediated by increased gastric acid, pepsinogen secretion and stimulatory effects on vasopressin. The action on the cyclo-oxygenase pathway also increases the risk of GERD and PUD.[72] Nicotine causes smooth muscle relaxation by action of endogenous nitric oxide as a nonadrenergic noncholinergic neurotransmitter.[73] The decrease in tone of the colon and gastric motility and reduced lower esophageal sphincteric pressure might be the reason of increased incidence of GERD.[74]
There is an increased incidence of treatment resistant Helicobacter pylori infection in smokers. It potentiates the effects of toxins of H. pylori by its action on the gastric parietal cells.[75] This effect could be due to histamine mediated response of nicotine.
IMMUNOLOGICAL SYSTEM
Nicotine has been known to be immunosuppressive through central and peripheral mechanisms. It impairs antigen and receptor mediated signal transduction in the lymphoid system leading to decreased immunological response. The T-cell population is reduced due to arrest of cell cycle. Even the macrophage response, which forms the first line defense against tuberculosis becomes dysfunctional and causes increased incidence of tuberculosis.[76] The migration of fibroblasts and inflammatory cells to the inflamed site is reduced. There is decreased epithelialization and cell adhesion and thus there is a delayed wound healing as well as increased risk of infection in nicotine exposed individuals.
The action on the hypothalamo-pituitary adrenal axis and autonomic nervous system stimulation via sympathetic and parasympathetic pathways affects the immune system. The adrenocorticotropic hormone (ACTH) secretion pathway and corticotrophin release is affected and this causes immunosuppression.[77]
OCULAR SYSTEM
Nicotine promotes pathologic angiogenesis and retinal neovascularization in murine models. It causes age-related macular degeneration in mice.[78] In a clinical study, the most virulent form of age-related maculopathy was associated with retinal neovascularization that contributed to visual deterioration. Tobacco smokers are known to be at greater risk of age-related macular degeneration than are nonsmokers.[79] In animal model, spraguely Dawley rats with type 1 diabetes treated with nicotine, developed cataract.[80] Thus the syngergistic relationship between nicotine and glucose metabolism exaggerating diabetes might cause accelerated cataract formation. There is synergistic relationship between nicotine and glucose metabolism which increases the risk of diabetes mellitus. This might cause accelerated cataract formation.
RENAL SYSTEM
Risk of chronic kidney disease in smokers is high. Cigarette smoking has been found to increase albumin excretion in urine, decrease glomerular filtration rate, causes increased incidence of renal artery stenosis and is associated with an increased mortality in patients with end-stage renal disease. The pathogenesis of renal effects is due to the action of nicotine via COX-2 isoform induction. The COX-2 isoforms causes increased glomerular inflammation, acute glomerulonephritis and ureteral obstruction.[81] There is impaired response of kidneys to the increased systemic blood pressure in smokers. This loss of renoprotective mechanism in smokers also leads to pathogenetic effects of nicotine on the renal system.[82]
REPRODUCTIVE SYSTEM – MALES
Nitrous oxide liberated from parasympathetico-nergic nerves plays a pivotal role in generating immediate penile vasodilatation and corpus cavernosum relaxation, and NO derived from endothelial cells contributes to maintaining penile erection. Nicotine causes impairment of NO synthesis. This may lead to loss of penile erections and erectile dysfunction.[83]
Various animal studies suggest that nicotine causes seminiferous tubules degeneration, disrupts the spermatogenesis and at cellular level, affect germ cell structure and function in males.[84] It decreases testosterone levels which is secondary to decreased production of StAR.[85] StAR is the protein which plays an important role in testosterone biosynthesis.
REPRODUCTIVE SYSTEM – FEMALE
Menstrual cycle
Nicotine by inhibiting the 21 hydoxylase causes hypoestrogenic state. It shunts the metabolites to formation of androgen. This leads to chronic anovulation and irregular menstrual cycles. Nicotine can predispose the endometrium to inappropriate cytokine production and irregular bleeding.[86] There is consistent evidence that increase in follicle-stimulating hormone levels and decreases in estrogen and progesterone that are associated with cigarette smoking in women, is atleast in part due to effects of nicotine on the endocrine system.[26]
Effect on oocytes
Nicotine affects the ovaries and alters the production of oocytes in various animal studies. Nicotine-treated oocytes appeared nonspherical with rough surface and torn and irregular zona-pellucida. Nicotine also caused disturbed oocyte maturation. There is a decreased blood flow to the oviducts and thus impaired fertilization.[87]
Peri-natal effects
Maternal smoking has always been known to have deleterious effects on the fetal outcome. There is an increased incidence of intrauterine growth restriction, still birth, miscarriages and mental retardation.[88] Various animal studies show retarded fetal growth and lower birth weight when treated perinatally with nicotine. The lower levels of ACTH and cortisol due to nicotine are probable reasons for the incidence of lower birth weight in the newborns.[89]
Maternal as well as grand maternal smoking has been found to increase risk of pediatric asthma. Another serious and important effect is the transgenic transmission of the addictive pattern.[29]
CONCLUSION
Nicotine is the fundamental cause of addiction among tobacco users. Nicotine adversely affects many organs as shown in human and animal studies. Its biological effects are widespread and extend to all systems of the body including cardiovascular, respiratory, renal and reproductive systems. Nicotine has also been found to be carcinogenic in several studies. It promotes tumorigenesis by affecting cell proliferation, angiogenesis and apoptotic pathways. It causes resistance to the chemotherapeutic agents. Nicotine replacement therapy (NRT) is an effective adjunct in management of withdrawal symptoms and improves the success of cessation programs. Any substantive beneficial effect of nicotine on human body is yet to be proven. Nicotine should be used only under supervision of trained cessation personnel therefore its sale needs to be strictly regulated. Needless to say, that research for safer alternative to nicotine must be taken on priority.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

