Histopathological pattern of lymphomas and clinical presentation and outcomes of diffuse large B cell lymphoma: A multicenter registry based study from India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 299-304
DOI: DOI: 10.4103/0971-5851.125250
Abstract
Context: The distribution of various subtypes of lymphomas in India is different from other parts of the world. There is scarce multicentric data on the pattern and outcomes of lymphomas in India. Aims: The aim of this study is to evaluate the histopathological and the clinical pattern and treatment outcomes of lymphomas in India based on the retrospective data collected from a multicenter registry. Materials and Methods: Retrospective data was collected at 13 public and private hospitals in India for patients diagnosed with lymphoma between January 2005 and December 2009. The data collection was performed in the setting of a multicenter lymphoma registry Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. Results: Non-Hodgkin′s lymphoma (NHL) constituted 83.17% and Hodgkin′s lymphoma (HL) for 16.83% of the 1733 registered and analyzed cases. Diffuse large B cell lymphoma (DLBCL) was the most common NHL (55%) followed by follicular lymphoma (11%). CHOP was the most common chemotherapy regimen administered (84%) while rituximab was used in 42.7% of those with DLBCL. Survival analysis of treatment naïve DLBCL patients (n = 791) was performed. Of these, 29% were lost to follow-up, 20% with active disease. The median follow-up in surviving patients is 31 (range: 1-88) months. Median progression-free survival (PFS) and overall survival (OS) in DLBCL patients has not reached. There was no significant difference in median PFS (69 months vs. 61 months, P = 0.1341), but OS was significant not reached (NR) vs. NR, P = 0.0012) within international prognostic index high or intermediate subgroups. Rituximab use was associated with significantly prolonged PFS (NR vs. 82 months, P = 0.0123), but not OS (NR vs. NR, P = 0.2214). Cox regression analysis in treatment naïve DLBCL patients showed a performatnce status, stage and receipt of six or more cycles of chemotherapy to be significantly associated with OS and all of the preceding plus rituximab use significantly associated with PFS. Conclusions: Our analysis confirms previous reports of distribution of lymphoma subtypes in India and suggests that patients who are able to receive the full course of chemotherapy achieve a better outcome. This indicates the importance of ensuring compliance to treatment utilizing various measures including patient and family counseling. Prospective studies are required to confirm these findings.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
The distribution of various subtypes of lymphomas in India is different from other parts of the world. There is scarce multicentric data on the pattern and outcomes of lymphomas in India.
Aims:
The aim of this study is to evaluate the histopathological and the clinical pattern and treatment outcomes of lymphomas in India based on the retrospective data collected from a multicenter registry.
Materials and Methods:
Retrospective data was collected at 13 public and private hospitals in India for patients diagnosed with lymphoma between January 2005 and December 2009. The data collection was performed in the setting of a multicenter lymphoma registry Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test.
Results:
Non-Hodgkin's lymphoma (NHL) constituted 83.17% and Hodgkin's lymphoma (HL) for 16.83% of the 1733 registered and analyzed cases. Diffuse large B cell lymphoma (DLBCL) was the most common NHL (55%) followed by follicular lymphoma (11%). CHOP was the most common chemotherapy regimen administered (84%) while rituximab was used in 42.7% of those with DLBCL. Survival analysis of treatment naïve DLBCL patients (n = 791) was performed. Of these, 29% were lost to follow-up, 20% with active disease. The median follow-up in surviving patients is 31 (range: 1-88) months. Median progression-free survival (PFS) and overall survival (OS) in DLBCL patients has not reached. There was no significant difference in median PFS (69 months vs. 61 months, P = 0.1341), but OS was significant not reached (NR) vs. NR, P = 0.0012) within international prognostic index high or intermediate subgroups. Rituximab use was associated with significantly prolonged PFS (NR vs. 82 months, P = 0.0123), but not OS (NR vs. NR, P = 0.2214). Cox regression analysis in treatment naïve DLBCL patients showed a performatnce status, stage and receipt of six or more cycles of chemotherapy to be significantly associated with OS and all of the preceding plus rituximab use significantly associated with PFS.
Conclusions:
Our analysis confirms previous reports of distribution of lymphoma subtypes in India and suggests that patients who are able to receive the full course of chemotherapy achieve a better outcome. This indicates the importance of ensuring compliance to treatment utilizing various measures including patient and family counseling. Prospective studies are required to confirm these findings.
INTRODUCTION
World-wide, the incidence of non-Hodgkin's lymphoma (NHL) is increasing faster than any non-cutaneous malignancy.[1] Globally, NHL incidence rates vary as much as five fold and lowest rates are reported from Asia;[2] the highest incidence rates are reported from United States and Europe. The frequency of various subtypes of NHL also differs in various regions.
National Cancer Registry Program by Indian Council of Medical Research has reported age adjusted rate (AAR) of NHL from 21 cities and towns. Highest AAR is from Imphal (6.8/100000) and lowest from Barshi (1.8/100000).[3] The pattern of NHL in India was first reported from AIIMS, New Delhi, India and showed that high grade lymphoma was the most common subtype (44.2%) followed by intermediate (39%) and low grade lymphomas (12.2%).[4] A single center study reported the distribution and clinicopathological characteristics of adult NHL presenting over a 1 year period.[5] B-and T-cell NHL constituted 79.3% and 18.8% of cases respectively and diffuse large B cell lymphoma (DLBCL) was the most common subtype (50.2%). Lower frequencies of follicular lymphoma (FL), marginal zone lymphoma, mantle cell lymphoma (MCL), peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and extranodal NK/T-cell lymphoma were seen compared with other Asian countries while higher frequencies of DLBCL and precursor T-lymphoblastic leukemia/lymphoma were noted. Extranodal and bone marrow involvement in MCL and PTCL-NOS were less frequent. Although these and other Indian studies[5,6,7] indicate differences in clinical and histopathological patterns of lymphomas in India, the data is limited and not nationally representative, especially with respect to the outcomes of treatment.
A Lymphoma Registry was established to understand the clinical, pathological and treatment patterns in lymphoma patients presenting to collaborating centers and their long-term outcomes. This is the first report of this registry that especially emphasizes the results in patients with DLBCL.
MATERIALS AND METHODS
Registry
The Lymphoma Registry was formed in August 2010 by 13 oncology institutions\hospitals in India [Figure 1] belonging both to public and private sectors. This enabled data to be collected on a continuum of socio-economic strata from low to high. The registry centers were distributed across various regions of the country and collectively likely to be representative of the Indian population. The objectives of establishing the registry were to study the clinical and pathological patterns and treatment outcomes of lymphoma patients in India. The data for this registry was collected using a proprietary Access Based Software (R Lymph 20) Priya Venkatadesikan, The Registrar of Trade Mark. This software was developed and managed by the lead author and Principal Investigator of the Registry. Data elements were included in the software in order to logically collect information on demographic characteristics, histopathological subtype, stage, treatment regimen, survival and important adverse effects in lymphoma patients. The collaborating centers/investigators provided important feedback prior to finalization of the software.
| Fig. 1 Lymphoma registry centers
Data collection
Retrospective data collection was undertaken by assessment of medical case records of lymphoma patients treated at each registry center from January 2005 to December 2009. The data of all patients who had received at least one cycle of chemotherapy for newly diagnosed lymphoma was collected and that of patients who had received prior chemotherapy was not included.
Study design and analysis
This is a retrospective analysis of patients diagnosed and treated with lymphomas at the collaborating centers of the registry from January 2005 to December 2009. The primary analyses are descriptive reports of the clinical and pathological characteristics of the patients and their tumors and survival outcomes in those with DLBCL. Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. Cox regression analysis was used to compare the effect of various factors on survival in a multivariate model. Patients were censored at the time of their last follow-up date. Progression free survival (PFS) was defined as the time interval from diagnosis until disease progression or relapse and overall survival (OS) was defined as the time interval from diagnosis until death. MedCalc, Medcalc Software, bvba, Belgium, and NCSS statistical packages were used for analysis.
The study was conducted after approval by respective institutional\independent ethics committees as applicable.
RESULTS
Patterns of lymphomas
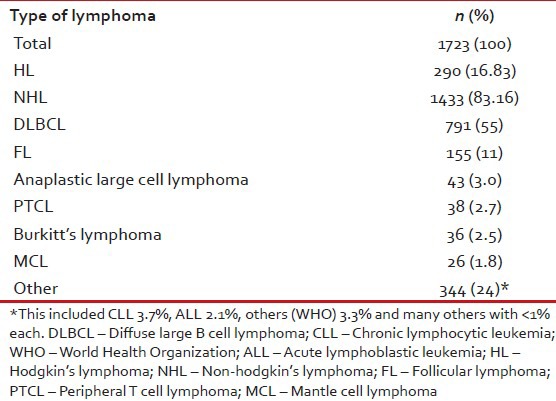
From October 2010 to June 2012, data was collected on 1723 lymphoma patients who were treated between January 2005 and December 2009 at 13 centers across India. There were 290 (16.83%) Hodgkin's lymphomas (HL) and 1433 (83.16%) NHL. The most common pathological subtypes of NHL were DLBCL (55%) and FL (11%). The other noteworthy subtypes of NHL were anaplastic lymphoma (3%), PTCL (2.7%), Burkitt's lymphoma (2.5%) and MCL (1.8%) [Table 1].
Table 1
Distribution of subtypes of lymphoma
DLBCL
The data of DLBCL patients in the registry is separately reported here. Other lymphoma subtype data will be reported in subsequent publications.
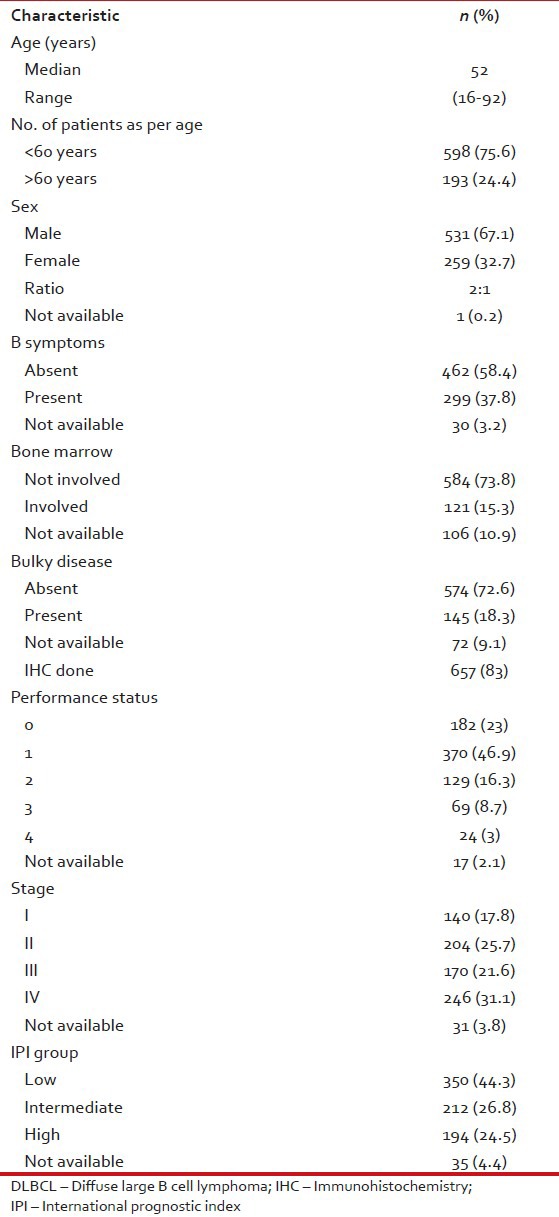
The patient characteristics are shown in Table 2. The median age was 52 years. We are only publishing here the results of patients more than 16 years of age (range: 16-92). Male to female ratio was 2:1, a majority of patients (70%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, 31.1% were diagnosed with stage IV disease, 44.3% had low international prognostic index (IPI) score, 37.8% reported B symptoms at presentation, 18.3% patients had bulky disease at initial presentation and bone marrow was involved in 15.3%. Immunohistochemistry was done in 83% patients.
Table 2
Patient characteristics of DLBCL patients
Treatment pattern
The standard regimen of cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) was the most commonly prescribed treatment (84%), while 42.7% of patients received rituximab in addition to chemotherapy. The median number of chemotherapy cycles was six (range: 1-12). Data on chemotherapy dose modification was available in 88% of patients and of these dose reduction was done in 9% of patients and 12% of patients discontinued chemotherapy. Radiation therapy was given to 36% of patients.
Outcome of DLBCL patients
At a median follow-up of 31 months (range: 1-88 months), 47% of patients were alive with no evidence of disease and 8% were alive with disease. Deaths were reported in 14% patients. Nearly 9% patients were lost to follow-up in remission and 20% were lost to follow-up with evidence of active disease at the time of the last evaluation.
Response rates
After completion of the first line therapy, 431 (55%) patients achieved a complete response (CR), 88 (11%) achieved a partial response (PR) and 203 (25%) reported not evaluated.
Survival
The progression-free and OS in all DLBCL patients is shown in Figures Figures22 and and33 respectively. The median PFS and OS are not reached and the 2-year PFS and OS are 75% and 79% respectively.
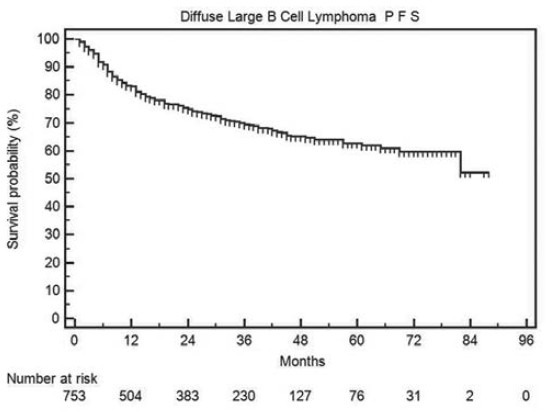
| Fig. 2 Progression-free survival in all diffuse large B cell lymphoma patients
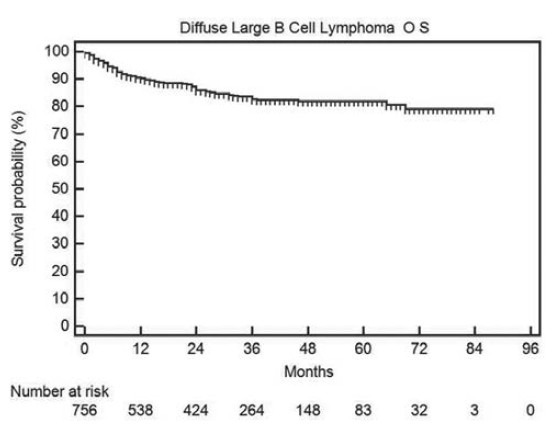
| Fig. 3 Overall survival in all diffuse large B cell lymphoma patients
Prognostic factors
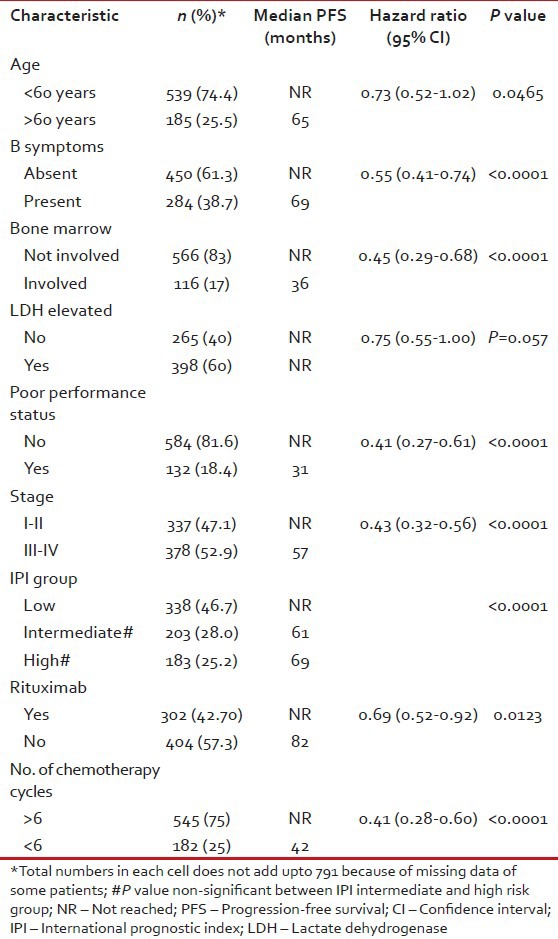
In univariate analyses of PFS, age >60 years, presence of B symptoms, bone marrow involvement, poor performance status (3-4) and advanced stage (≥III) were significant adverse factors [Table 3]. It is noteworthy that PFS in low risk IPI group patients was significantly superior to intermediate (P = 0.0005) and high (P < 0.0001) risk patients, but there was no difference between intermediate and high risk IPI patients [Figure 4]. Median PFS has not been reached in patients with Stage I and II, whereas it was 57 months in patients with Stage III and IV disease [Figure 5].
Table 3
Univariate analysis of prognostic factors
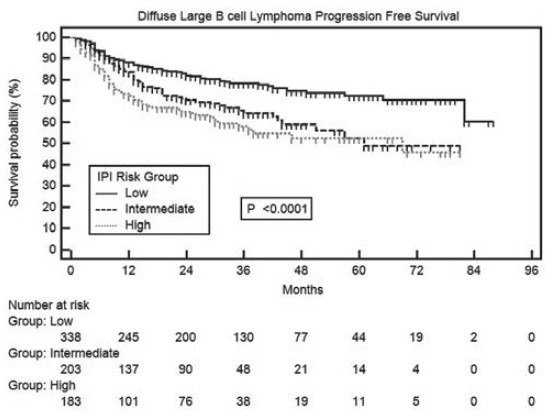
| Fig. 4 Progression-free survival in diffuse large B cell lymphoma patients in international prognostic index risk groups
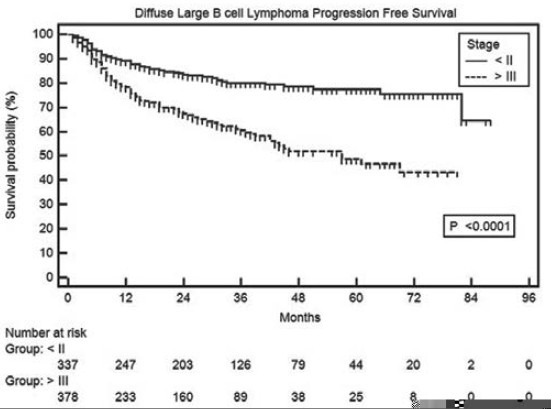
| Fig. 5 Progression-free survival in diffuse large B cell lymphoma patients as per modified Ann Arbor stages
Impact of Rituximab and number of chemotherapy cycles
The median PFS has not been reached in patients who received rituximab, whereas it was 82 months in those who didn’t receive this drug (P = 0.0123). However, this PFS benefit did not convert into prolongation of OS (P = 0.22). the median PFS has not been reached in patients receiving more than or equal to six chemotherapy cycles, whereas it was 42 months in those who received less than six cycles (1-5) (P < 0.0001).
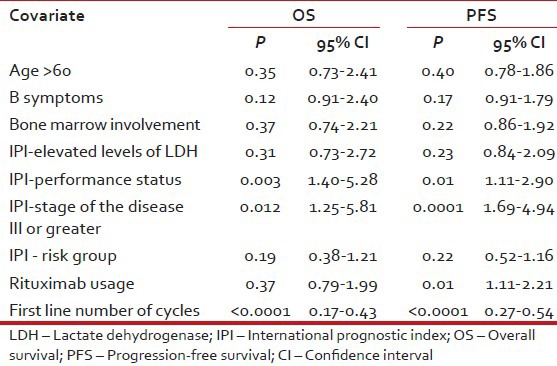
In multivariate analysis of treatment naïve DLBCL patients, significant variables associated with adverse PFS were poor performance status, higher stage, use of less than six cycles of chemotherapy and non-use of rituximab whereas adverse prognostic factors for OS were all the preceding except rituximab non-use [Table 4].
Table 4
Multivariate analysis for OS and PFS
DISCUSSION
Geographic variations in lymphoid malignancies are well-known, but data from India is limited. Individual centers have published their experience,[6,7] but no nationwide registry data has been published until now. Our lymphoma registry is the first multicentric effort in collecting, analyzing and publishing the patterns of care and outcome data of lymphoma patients in India. Such coordinated activities are challenging in a country like India where oncologists and cancer centers are burdened with huge patient load in diverse conditions. The current effort is proof of concept of the feasibility and reliability of a nationwide, multicenter, patterns of care and outcome type of registry for cancers.
The distribution of pathological subtypes of lymphoma (NHL vs. HL) in our registry is consistent with other reports from India[7,8] and somewhat higher for NHL compared to a rural registry.[8] Within NHL the preponderance of DLBCL followed by FL in our report is also consistent with other reports from India.[5,6,7,8] Median age of our DLBCL population was 52 years, which is younger compared with that seen in Western populations,[9,10] but is consistent with most other reports from India.[6,11] The younger average age of our patients is also consistent with the pattern seen in most other malignancies in India and is likely due to the effect of a younger population pyramid in this country. B symptoms were present in 37% DLBCL patients and have been variably reported from other reports from this country.[6,11] ECOG performance status of 0-I are 70% in our study, which is higher when compared to published data from a tertiary center.[12] This could be due to the data from a mix of private and public institutions.
Our survival data appears to be high possibly due to a significant proportion of patients lost to follow-up and censored at that point as a non-event. Our results show that low IPI score is a reliable prognostic indicator in DLBCL, but there was no difference between intermediate and high risk patients. This is in contrast to most reports from Western countries, which show a robust prognostic significance between the three groups. The reasons for this discrepancy are unclear.
As with other expensive medications, the use of rituximab was low in our population (42.7%), but is increasing compared to reports from earlier years.[13] The addition of rituximab to chemotherapy resulted in superior PFS, but not OS in our analysis, which is in contrast to reports from randomized trials.[14,15] Although the reasons for this result are unclear it is possible that features of retrospective analyses like considerable fraction being lost to follow-up, imbalance in numbers in the two groups and other systematic biases could be explanatory. It is also possible that with increasing access to rituximab over the years in India, a considerable fraction of the first line rituximab naïve patients received it after relapse, blurring the OS difference. Whatever be the reason, our analysis cannot negate the proven benefit of rituximab as a component of the first line treatment regimen in DLBCL.
In our study, 25% of patients received < 6 cycles of chemotherapy. Similarly, a high percentage of patients are reported who have taken < 6 cycles in Prakash et al.[12] study from a tertiary cancer center in India. Our results are also suggest that receipt of an adequate regimen of at least six cycles of frontline chemotherapy was associated with a superior outcome. The four main reasons for patients receiving less than six cycles of treatment could be low stage disease, severe adverse effects of chemotherapy and non-compliance to treatment and resistance to the first line (mainly CHOP) regimen, the last two of which could be responsible for the poor outcome in these patients. In the absence of detailed data on the reasons for receipt of less number of cycles, it is not possible to be definitive about the contribution of non-compliance to this result. However, it is likely that it contributed in at least some measure to the poor outcome in this subgroup, because of a variety of factors such as financial and logistic constraints in our population. It will be important to study this factor in prospective studies because it is a potentially correctable cause of inferior outcomes of treatment.
Our study has several weaknesses mainly related to its retrospective nature. It is possible that data collected from the case charts was neither complete nor entirely accurate. Moreover, there is likely to have been some pathological misclassification because of the variable expertise and infrastructure available at different centers. There were a relatively large number of patients who were lost to follow-up.
Despite these caveats, we believe that our analysis is a valuable contribution to the lymphoma literature from this part of the world. A valuable outcome has been the establishment of feasibility of systematic data collection by a large group of oncologist, spread across the country, together. We hope this will encourage others to form groups and collect data prospectively in various malignancies.
ACKNOWLEDGMENT
The authors would like to thank the funding for the establishment and running of lymphoma registry was provided by unrestricted educational grant from Roche Products India Pvt. Ltd. The authors are grateful acknowledge RPIPL for their support and special thanks to Dr. Anil Kukreja and Dr. Rupesh Pophale for assisting in manuscript writing.
Footnotes
Source of Support: Lymphoma registry established with unrestricted educational grant from Roche Products (India) Pvt. Ltd.
Conflict of Interest: Registry members received honorarium from Roche as advisory board members.
REFERENCES

| Fig. 1 Lymphoma registry centers

| Fig. 2 Progression-free survival in all diffuse large B cell lymphoma patients

| Fig. 3 Overall survival in all diffuse large B cell lymphoma patients

| Fig. 4 Progression-free survival in diffuse large B cell lymphoma patients in international prognostic index risk groups

| Fig. 5 Progression-free survival in diffuse large B cell lymphoma patients as per modified Ann Arbor stages


 PDF
PDF  Views
Views  Share
Share

