HIV-associated hematologic malig
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 141-145
DOI: DOI: 10.4103/0971-5851.190355
Abstract
Context and Aim: Data on HIV associated hematologic malignancies is sparse from India. This study attempts to analyze the spectrum and features of this disease at a tertiary cancer center in India. Setting and Methods: Retrospective study from case records of patients registered with a diagnosis of hematologic malignancy and HIV infection between January 2010 and June 2015. Results: Thirteen cases of HIV associated hematologic malignancies were identified, six of them pediatric. HIV diagnosis was concurrent to diagnosis of cancer in 12 and preceded it in one of them. ECOG PS at presentation was >1 in all of them. All patients, except one, had B symptoms. Six of the patients had bulky disease and six are stage 4. Predominant extranodal disease was seen in 67% of them. NHL accounted for 10 of 13 patients and DLBCL-Germinal center was the most common subtype. Mean CD4+ cell count was 235/μL (range, 32-494). HAART could be given along with chemotherapy to 11 patients. Two-thirds of patients received standard doses of therapy. Chemo-toxicity required hospitalization in 58%. CR was achieved in 45% and 36% had progressive disease with first-line therapy. At the time of last follow up, 3 patients were alive with responsive disease, 2 in CR and 1 in PR. None of the pediatric patients were long time responders. Conclusions: These malignancies were of advanced stage and higher grade. Goal of therapy, in the HAART era, is curative. Pediatric patients had dismal outcome despite good chemotherapy and HAART. There is an urgent need to improve data collection for HIV related cancers in India.
Keywords
AIDS-related lymphoma - diffuse large B-cell lymphoma - highly active anti-retroviral therapy - HIV-related cancer in India - pediatric HIV malignanciesPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context and Aim:
Data on HIV associated hematologic malignancies is sparse from India. This study attempts to analyze the spectrum and features of this disease at a tertiary cancer center in India.
Setting and Methods:
Retrospective study from case records of patients registered with a diagnosis of hematologic malignancy and HIV infection between January 2010 and June 2015.
Results:
Thirteen cases of HIV associated hematologic malignancies were identified, six of them pediatric. HIV diagnosis was concurrent to diagnosis of cancer in 12 and preceded it in one of them. ECOG PS at presentation was >1 in all of them. All patients, except one, had B symptoms. Six of the patients had bulky disease and six are stage 4. Predominant extranodal disease was seen in 67% of them. NHL accounted for 10 of 13 patients and DLBCL-Germinal center was the most common subtype. Mean CD4+ cell count was 235/μL (range, 32-494). HAART could be given along with chemotherapy to 11 patients. Two-thirds of patients received standard doses of therapy. Chemo-toxicity required hospitalization in 58%. CR was achieved in 45% and 36% had progressive disease with first-line therapy. At the time of last follow up, 3 patients were alive with responsive disease, 2 in CR and 1 in PR. None of the pediatric patients were long time responders.
Conclusions:
These malignancies were of advanced stage and higher grade. Goal of therapy, in the HAART era, is curative. Pediatric patients had dismal outcome despite good chemotherapy and HAART. There is an urgent need to improve data collection for HIV related cancers in India.
INTRODUCTION
With the introduction and widespread availability of highly active anti-retroviral therapy (HAART), the landscape of HIV/AIDS has changed considerably. This is true for HIV-associated malignancies as well. Post-HAART, the incidence of Kaposi's sarcoma and central nervous system (CNS) lymphoma (among AIDS-defining cancers) decreased in parallel with AIDS-defining infections. On the other hand, the incidence of systemic non-Hodgkin's lymphoma (NHL) and cervical cancer decreased less than others and remains higher in HIV-infected patients than in the general population. Currently, malignancies are the most frequent underlying cause of death (around one-third) of HIV-infected patients.[1]
The situation in a developing country like India is suboptimal. Lack of widespread availability of diagnostic facilities, access to HAART, and cancer therapy were major limitations. Currently, India has the 2nd highest number of people with HIV/AIDS (PWHA). National AIDS Control Organization (NACO), the nodal agency to control the spread of HIV/AIDS in India, estimates the current prevalence of HIV infection to be 0.27%.[2] The assessment of problem load of HIV-associated malignancies in India is limited by lack of quality epidemiologic data collected in this regard.
The current study is an attempt to study the spectrum of hematologic malignancies encountered in HIV patients in the Department of Medical Oncology of a Tertiary Cancer Center in India. Clinical features, treatment factors, and outcomes were assessed.
MATERIALS AND METHODS
We have retrospectively reviewed the records of patients with hematologic malignancies registered in our clinics from January 2010 to June 2015. Records of patients who were known HIV positive or were detected to be HIV positive during initial evaluation were retrieved. Data of epidemiologic, clinical, pathologic, staging, therapy, and response assessment factors, as well as outcome and long-term follow-up, were recorded.
RESULTS
A total of 13 patients with a diagnosis of HIV infection along with a hematologic malignancy were identified. The baseline patient characteristics were depicted in Table 1. The age range was 4–56 years with a mean of 28.3 (standard deviation: 20.2). Six patients were of pediatric age group (>18 years) with a mean age of 8.5 years. Only one female patient was encountered. NHL accounted for 10 of the 13 patients. Diffuse large B-cell lymphoma (DLBCL) was the single most common histology (7 patients, 54%). Most of the DLBCL were germinal center (GC) type (5 out of 7, 71%).
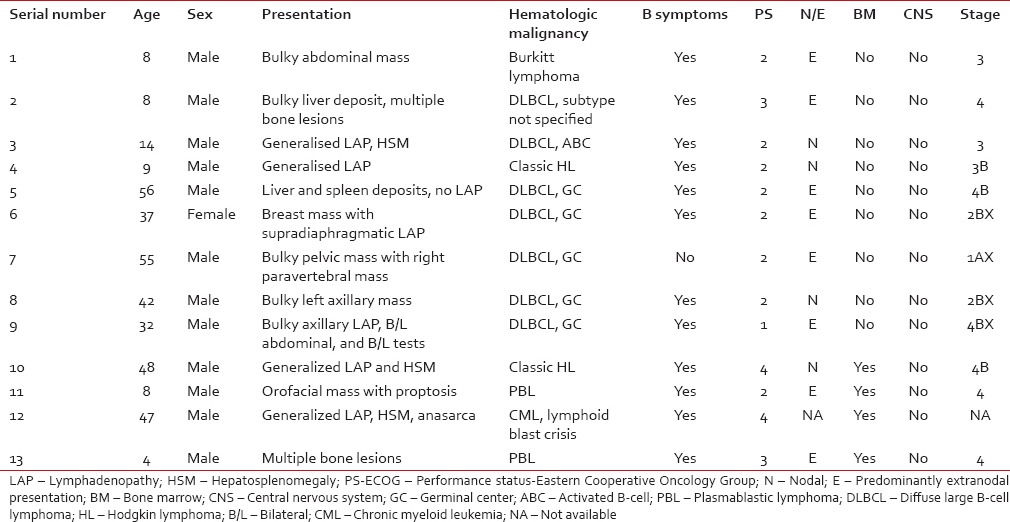 All patients, except one, had B symptoms at presentation. Among lymphomas, the majority of patients had Stage 4 disease (6 out of 12, 50%). Six patients (50%) had bulky disease. Eight patients (67%) had predominantly extranodal disease. Eastern Cooperative Oncology Group performance status at the presentation of all patients, except one, is more than 1. Bone marrow was involved in 31% (four out of 13). None of the patients had CNS involvement. Data on serum lactate dehydrogenase (LDH) levels were available for nine patients, with a mean of 728 IU/L (range, 238–1526).
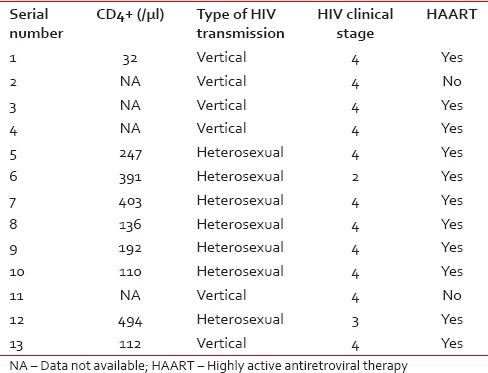
All patients, except one, had B symptoms at presentation. Among lymphomas, the majority of patients had Stage 4 disease (6 out of 12, 50%). Six patients (50%) had bulky disease. Eight patients (67%) had predominantly extranodal disease. Eastern Cooperative Oncology Group performance status at the presentation of all patients, except one, is more than 1. Bone marrow was involved in 31% (four out of 13). None of the patients had CNS involvement. Data on serum lactate dehydrogenase (LDH) levels were available for nine patients, with a mean of 728 IU/L (range, 238–1526).Data regarding HIV status of the patients was depicted in Table 2. The diagnosis of HIV was made at the time of evaluation for hematologic malignancy in all of the patients except one. This was a case of lymphoma involving breast where the patient had HIV for 4 years before diagnosis of NHL. CD4+ cell count at the time of diagnosis of cancer was available for nine patients, with a mean of 235/µL (range, 32–494). Data of anti-HIV therapy were available for 12 out of whom 11 took HAART and one refused.
Table 2
HIV infection characteristics of the patients
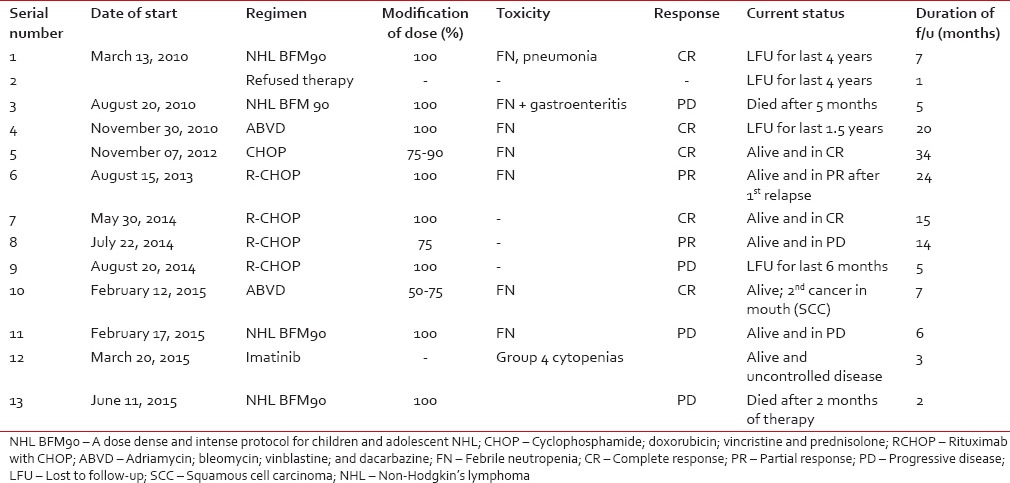
Out of the 13 patients, 12 were administered anti-cancer therapy while one patient refused therapy. Pediatric NHL patients were treated with NHL BFM 90 protocol while adult NHL patients received cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) based chemotherapy. Rituximab was given to four of five adult NHL patients. Chemotherapy was given in standard doses, at least from 2nd cycle onward, in eight of 12 patients (67%) who received therapy. One patient of chronic myeloid leukemia (CML) lymphoid blast crisis was given suboptimal therapy owing to poor social support. Chemotherapy-related toxicity requiring hospitalization occurred in seven out of 12 patients (58%). Response to first-line therapy was evaluable in 11 of 13 patients, with five of them (45%) achieving a complete response (CR). Four patients (36%) had progressive disease on 1st line therapy. At the time of the last follow-up, three out of 13 (23%) had a responsive disease, two in CR and one in partial response (PR). Two patients have succumbed to their disease, and four patients were lost to follow-up. Three patients had progression of disease and one patient of Hodgkin's lymphoma (HL) developed a 2nd malignancy (oral squamous cell cancer). The mean duration of follow-up in our outpatient department for adult patients (>18 years) was 14.6 months (range, 3–34). The same for pediatric patients was 6.8 months (range, 1–20). Treatment-related data were shown in Table 3.
Table 3
Treatment and outcomes
DISCUSSION
India contributes to a significant portion of global HIV burden, with the latest NACO report putting it at approximately 2.1 million people living with HIV/AIDS (PWHA). However, data assessing the problem load of HIV-associated malignancies is very underwhelming from this nodal agency of cancer control in India. Data from the western countries point that about one-third of HIV patients suffer from cancer.[1] The focus in a developing country like India was more on tuberculosis and other opportunistic infections which are major threats to people living with HIV/AIDS (PWHA). In a study by Sharma et al. of 135 consecutive PWHA admitted at a north Indian hospital, TB was the most specifically identified illness accounting for 71%.[3] With the availability of HAART and treatment and prevention of opportunistic infections, an increase in life expectancy of HIV-infected individuals and an increase in HIV-related malignancies is expected.[4]
Dhir et al. studied cancer pattern in patients registered at their HIV cancer clinic from 2001 to 05 and calculated site-specific population incidence ratio (PIR). NHL had the highest PIR followed by anal canal cancer and HL.[5] HIV infection was found in 1.2% of cancer admissions during this period. In a study of spectrum of malignancies in HIV infected south Indian adult patients from 1998 to 2008, 42 cases of HIV-associated malignancies were noted.[6] NHL (38%) and HL (17%) accounted for the majority of these cancers. No case of Kaposi sarcoma was seen in these bigger reports from India. Indian studies specifically on hematologic malignancies associated with HIV/AIDS are also very few. The types of lymphoma occurring among PWHA seen at Tata Memorial Hospital, Mumbai, have been examined by Agarwal et al.[7] Among 35 PWHA with hematologic malignancies, they found 24 NHL, seven HL, and four plasmacytomas. Sharma et al. have reported a case series of seven patients of NHL from a Tertiary Cancer Center in North India.[8] Sachdeva et al., in a retrospective analysis of 5100 HIV-infected individuals from 2004 to 2009, found hematologic malignancies in 14 patients (0.3%).[9]
Hematologic malignancies added 2019 new cases of cancer in the year 2010 as per the Delhi Cancer Registry. This contributes to 11.7% of all newly diagnosed cases.[10] Our center accounts for 22% of this caseload. The proportion of HIV positivity in hematologic malignancies could not be estimated owing to lack of systematic recording of such data, providing an initiative to do this study.
NHL, like in the rest of the studies,[11,12] was the most common diagnosis in this cohort. All of them are high-grade B-NHL. AIDS-related lymphoma (ARL) have an early age of onset, high extranodal disease load, bulky tumor size, high levels of LDH levels, and advanced clinical stage.[13,14] They were generally considered to have a poor prognosis.[13] We encountered similar clinical characteristics in our patients. We have seen a male to female ratio of 12:1 and this was similar to data from western countries.[15,16]
Plasmablastic lymphoma (PBL) is a rare category of ARL with a predilection for oral cavity.[17] Two of our pediatric patients had this aggressive disease, one of them with a large oropharyngeal mass. HL is increasing in incidence among HIV-associated cancers [18] and its proportion among Non-AIDS Defining Cancers is on rise even in the post-HAART era.[19] Acute and chronic leukemias also occur at an increased frequency in HIV-infected individuals than in non-HIV-infected population,[20] but they were generally rare. We had a case of CML who presented in lymphoid blast crisis.
Treatment decisions require an understanding of the patient's HIV disease as well as the curative potential of malignancy encountered.[20] Data on the HIV viral load are lacking in all of our patients and CD4 counts at the time of diagnosis was available for nine of 13 patients. None of our patients had HIV-related complications during therapy. This is possibly because the mean CD4+ cell count of this cohort was 238/µL and 12 of 13 were newly diagnosed with HIV. An important concern specific to HIV-related cancer management is what to do with HAART during chemotherapy. There are proponents for both stopping HAART during chemotherapy as well as continuation of HAART along with chemotherapy. The concerns were borne out of a potential for drug interactions, overlapping toxicities, lymphopenia that could further decrease CD4+ cell count and possible adherence problems.[20,21,22,23] The present consensus was to give HAART along with chemotherapy in patients who were otherwise fit to receive treatment.[20] Anti-cancer therapy, as well as HAART, was offered to all of our patients at the time of diagnosis. One patient has refused any form of therapy and was lost to follow-up within a month of diagnosis. The HAART regimen preferred at our center was combination of tenofovir, lamivudine, and efavirenz. These drugs do not have significant interactions with common chemotherapy regimens such as CHOP used to treat our patients. All patients were able to take HAART along with chemotherapy. Dose reductions of chemotherapy were not required owing to HAART in our cohort.
DLBCL was the most common histology in our cohort with majority being the GC type. The preferred regimen for this group as per the current available literature seems to be R-EPOCH, though phase 3 data was awaited.[23,24] All the adult patients in our subgroup were considered for CHOP-based chemotherapy. Rituximab was opted for by four of five patients, with the other not being able to afford the drug. This subset had good response with 80% of them achieving CR/PR. At the time of last follow-up, three of them were alive and with responsive disease at a mean follow-up duration of 19 months.
Outcomes of our pediatric cohort were dismal. Part of this could be explained by the fact that two cases were PBL, and one patient refused therapy. HIV-related PBL is an extremely difficult to treat subtype with a very poor prognosis.[25] Although both of our patients received high-dose pediatric protocol (NHL BFM90) along with HAART, they both had progressive disease on therapy underlining the poor responsiveness of this disease. CR rate was 40%, and none of the responses were long lasting. No long-term survivor with a responsive disease was there in our cohort with a mean follow-up of 6.8 months (range, 1–20) after diagnosis. Although it is difficult to draw conclusions from such a small treatment group, the results are still difficult to comprehend. Literature is also sparse and inconclusive.[26,27,28]
Outcomes of HIV-infected cancer patients were improving in the post-HAART era.[20,29] Concerns of increased mortality in this subset of patients seem more related to disparities in cancer treatment.[30] Previous studies from India in related cohorts [8,9] had early mortality or loss to follow-up and poor response to chemotherapy. Better results in the present group of patients, at least in the DLBCL subtype, could be attributed to good dosage of chemo-immunotherapy that could be achieved along with continuation of HAART.
CONCLUSIONS
We can draw few important conclusions from our study of this series of patients, despite its small size and limited follow-up. NHL is the most common of HIV-associated hematologic malignancies, with GC-DLBCL being its most common subtype. These are of higher grade, advanced stage and with a younger age at presentation. In the era of HAART, the goal of therapy has changed from palliation to cure. Timely initiation of HAART and effort to maintain good dose intensity of chemo-immunotherapy seem to be critical for good outcomes. There is a need to improve the collection of epidemiologic data for HIV-related cancers in India. This would pave way for devising rational treatment protocols better suited for the needs unique to this country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

