Hyponatremia of non-small cell lung cancer: Indian experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(03): 139-142
DOI: DOI: 10.4103/0971-5851.92810
Abstract
Background: Hyponatremia is a hazardous complication of lung cancer and its treatment. It is seen at presentation in approximately 15% of patients with small-cell lung cancer (SCLC) and 1% of patients with non-small cell lung cancer (NSCLC). Platinum compounds used as first-line agents along with taxols frequently cause hyponatremia. Till date there is no data on its prevalence in patients with advanced lung cancer in the Indian subcontinent. Aim: This study was undertaken to find out its incidence before and after institution of chemotherapy and to observe the results of treatment of hyponatremia in a group of lung cancer patient. Materials and Methods: Forty patients with advanced lung cancer (25 patients with stage III disease and 15 with stage IV disease) were included in the study. Variables looked at included, but were not limited to, serum sodium, serum albumin, serum alkaline phosphatase, serum lactate dehydrogenase, and hemoglobin. These variables were measured as per the standard clinical laboratory procedure. No ethics approval was required as these parameters are routinely measured in such patients. Results: In the chemo-naïve state, one out of five cases with SCLC (20%) had hyponatremia at presentation; among the 35 cases of NSCLC, 7 patients (20%) had hyponatremia at presentation, which is in sharp contrast to earlier reports of 1% prevalence of hyponatremia in this group. Among the 27 cases who died within 6 months, 11 had hyponatremia; this finding was statistically highly significant. Conclusion: In India, NSCLC patients are at high risk of having hyponatremia at presentation and this is significantly associated with a worse outcome.
Keywords
Hyponatremia - non-small cell lung cancer - syndrome of inappropriate secretion of antidiuretic hormonePublication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Hyponatremia is a hazardous complication of lung cancer and its treatment. It is seen at presentation in approximately 15% of patients with small-cell lung cancer (SCLC) and 1% of patients with non-small cell lung cancer (NSCLC). Platinum compounds used as first-line agents along with taxols frequently cause hyponatremia. Till date there is no data on its prevalence in patients with advanced lung cancer in the Indian subcontinent.
Aim:
This study was undertaken to find out its incidence before and after institution of chemotherapy and to observe the results of treatment of hyponatremia in a group of lung cancer patient.
Materials and Methods:
Forty patients with advanced lung cancer (25 patients with stage III disease and 15 with stage IV disease) were included in the study. Variables looked at included, but were not limited to, serum sodium, serum albumin, serum alkaline phosphatase, serum lactate dehydrogenase, and hemoglobin. These variables were measured as per the standard clinical laboratory procedure. No ethics approval was required as these parameters are routinely measured in such patients.
Results:
In the chemo-naïve state, one out of five cases with SCLC (20%) had hyponatremia at presentation; among the 35 cases of NSCLC, 7 patients (20%) had hyponatremia at presentation, which is in sharp contrast to earlier reports of 1% prevalence of hyponatremia in this group. Among the 27 cases who died within 6 months, 11 had hyponatremia; this finding was statistically highly significant.
Conclusion:
In India, NSCLC patients are at high risk of having hyponatremia at presentation and this is significantly associated with a worse outcome.
INTRODUCTION
Hyponatremia is a serious comorbidity in any illness and needs particular attention because of its asymptomatic presentation, difficult management, grave neurological consequences, and associated excess mortality.[1] In cancer patients, also, it poses a very serious challenge in management. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) and depletional states are the major causes of such hyponatremia in cancer patients and together are responsible for two-thirds of these cases. SIADH may be caused by vasopressin secreted by the tumors, abnormal secretory stimuli (e.g., intrathoracic infection, positive-pressure ventilation), or cytotoxicity affecting paraventricular and supraoptic neurons. Chemotherapy-induced renal salt wasting, gastrointestinal sodium and water loss, or tumor-induced salt wasting (mediated by atrial natriuretic peptide) may also cause hyponatremia.
Cancers of lung, breast, and head and neck are the ones most commonly associated with hyponatremia, which is predominantly of the SIADH type. Among these cancers, lung cancer has the lowest survival, the overall rate being only 9%–13% in comparison to 80% and 50% for breast and head and neck cancer, respectively.
The high mortality[1] associated with hyponatremia will adversely affect the already low survival rates in advanced lung cancer. Mortality in hyponatremia is in the range of 27%–42%.[1] The reasons are not known with certainty but status epilepticus or osmotic demyelination syndrome (which is also known as central pontine myelinolysis and is characterized by focal demyelination in the pons and extrapontine areas associated with serious neurologic sequelae) is observed in such cases. Hyponatremia can occur not only in untreated lung cancer but also during treatment. A very delicate balance is needed for its management, which is not very easy.
It is clear that hyponatremia in lung cancer is a very important issue. Primary carcinoma of the lung was an uncommon cancer until the 1930s. Now, it is the single most common cause of cancer-related deaths and is projected to cause three million deaths this year alone.[2] Two major histological types are predominant: Non-small cell lung cancer (NSCLC), which accounts for 85% of lung cancer cases, and small-cell lung cancer (SCLC), which accounts for 15%.
While the incidence of hyponatremia in lung cancer is reported to be only 1% in NSCLC and 15% in SCLC,[3] these are Western data. A study of hyponatremia in lung cancer has never been undertaken in India. Hence, we designed this study to find out the extent and implications of hyponatremia in lung cancer.
MATERIALS AND METHODS
Forty cases of advanced lung cancer (stage III - 25 cases and stage IV - 15 cases) were included in this study conducted at the Netaji Subhas Chandra Bose Cancer Research Institute, Kolkata, from July 2008 to December 2008. The subjects were free from gross liver and kidney disease and brain metastasis. Five cases of SCLC and 35 cases of NSCLC were diagnosed by fine needle aspiration cytology (FNAC) or directed biopsies. All cases underwent CT scan in screening, and the stage of disease was calculated and monitored according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 4.0).[4] All NSCLC cases were treated with 4–6 courses of paclitaxel and cisplatin with an interval of 21 days between courses. SCLC cases were treated with an etoposide and cisplatin regime. To treat hyponatremia we used oral salt, along with fluid restriction to 500 mL per day. In a few cases we used hypertonic saline. Workup for each patient consisted of clinical examination, blood counts, blood biochemistry, standard chest roentgenography, computed tomographic (CT) scan of chest and upper abdomen, fiberoptic bronchoscopy, and bone scan.
Five parameters were selected from among the routine tests and their association with the prognosis of the disease was examined. The parameters were: Serum sodium (Na), serum alkaline phosphatase (ALP), serum lactate dehydrogenase (LDH), serum albumin (Alb), and hemoglobin (Hb).
Serum sodium
IL 943 flame photometer (Instrumentation Laboratory, Milan, Italy), a Stat Profile M® direct ion-selective electrode (Nova Biomedical Corp., Waltham, MA), and a Hitachi 717 indirect ion-selective electrode (Hitachi Ltd., Tokyo, Japan) were used to measure serum Na. Each method was calibrated and performed according to the manufacturers’ specifications via internal (ISO 9001) and external (regional inter-laboratory) quality controls. Hyponatremia was defined as per the Common Terminology Criteria for Adverse Events (CTCAE v 4.0), with the following cutoffs: Grade 1: Lowest limit of normal (LLN)–130 mmol/L; grade 2: nil; grade 3: < 130–120 mmol/L; grade 4: < 120 mmol/L; grade 5: Life-threatening consequences or death. The normal range for serum Na as per our hospital lab is 135–145 mmol/L.
Serum alkaline phosphatase and serum lactate dehydrogenase
Serum ALP and serum LDH were measured by the kinetic UV method of DGKC (German Clinical Chemistry), using Roche Kit® (Swisslab GmbH, Berlin, Deutschland).
Serum albumin
Serum albumin (Alb) was measured by AssayMax Human Albumin ELISA Kit® (St. Charles, MO).
Serum osmolality, which can be measured directly via osmometry, was calculated in our study using the formula 2(Na) mEq/L + serum glucose (mg/dL)/18 + BUN (mg/dL)/2.8.
Statistics
Kaplan-Meier survival analysis with logrank test was done to show group differences and the significance (P < 0.05) of different parameters in patients who died and who lived at/after the sixth month.
RESULTS
The mean age of patients in our study was 49 years. Of the 40 cases, two were females. There were 5 cases of SCLC and 35 cases of NSCLC. They were all of advanced stages (>IIIA), with 25 cases of stage IIIB and 15 cases of stage IV). According to histological characteristics, 16 were predominantly adenocarcinoma, 11 were squamous cell carcinoma, 3 were of the large cell variety, and another 10 cases were of nonspecified type.
Among the 15 stage-IV cases, 5 had a second lesion in the lung, 4 had liver mets, and 3 each had retroperitoneal nodes and bone mets. Eastern Cooperative Oncology Group (ECOG) performance status was 0 in thirty-seven cases, 1 in two cases, and 3 in one case.
No acute case of hypernatremia was detected in our series. In the chemo-naïve condition, one case of SCLC and seven cases of NSCLC presented with hyponatremia. Among the seven NSCLC cases, two had grade III and the others had grade I hyponatremia (CTCAE v 4.0).[5] There was only one case of hyponatremia in our series where the osmolality was not low as calculated by the formula mentioned earlier. However, there were also three cases of very low-normal serum Na of 136 mEq/L, all of which went into the hyponatremic state very rapidly, before the second course of chemotherapy. Chemotherapy had a reversal effect on the hyponatremia in three cases but in the other cases the hyponatremia continued despite treatment. Hyponatremia generally appeared after the second course. In all, 15 cases developed hyponatremia, which was detected on 30 occasions during their treatment. Seven cases responded to oral salt intake and fluid restriction or hypertonic saline administration. A conspicuous finding was that among the 27 cases that died within 6 months, 11 had hyponatremia.
The range of the parameters in dead and living patients is shown in Figure 1. The results show that only serum Na was significantly associated with the worst prognosis of death (P < 0.03). The log-rank test for serum Na is shown in Figure 2. The other parameters did not have any significant association with death.
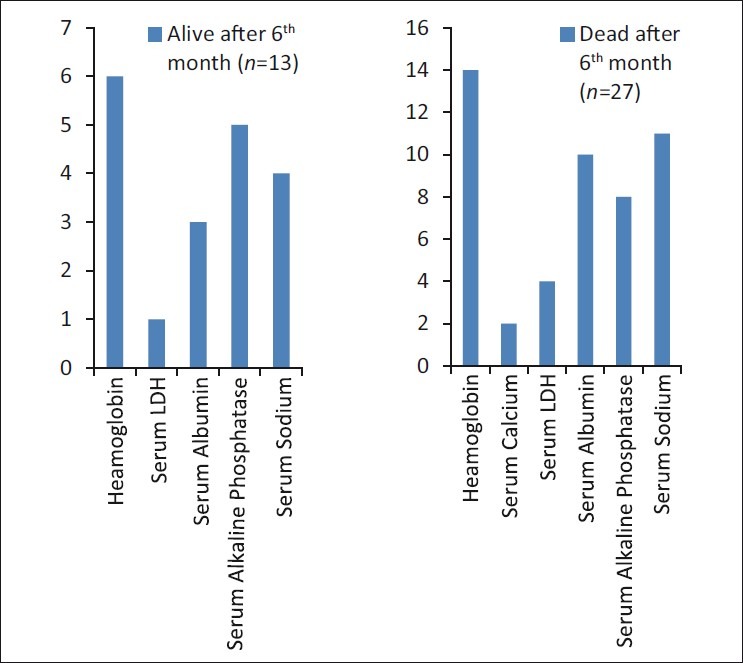
| Fig. 1 Range of parameters in living and dead patients
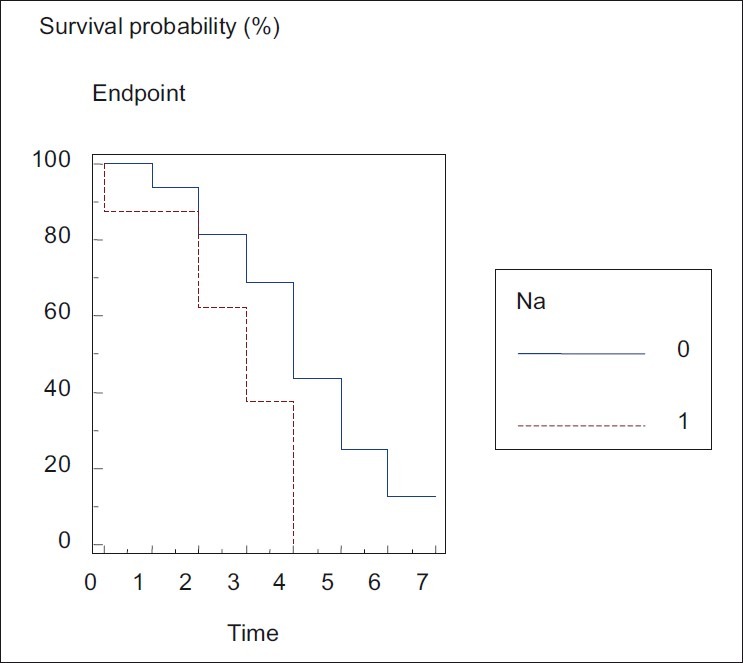
| Fig. 2 Logrank test for serum sodium
DISCUSSION
In 1957, Schwartz et al.[6] first described hyponatremia due to inappropriate secretion of antidiuretic hormone as a syndrome and the condition was consequently named as the Schwartz-Bartter syndrome in lung cancer.
Chute et al.[3,7] showed that hyponatremia occurs in approximately 15% of SCLC and 1% of NSCLC cases. Sørensen et al.[8] reported that SIADH occurs in 3% of patients with head and neck cancer, in 0.7% of NSCLC patients (3 cases out of 427), and in 15% of SCLC patients (214 cases out of 1473). The role of atrial natriuretic peptide[9] and chemotherapy in causing hyponatremia by tumor-induced or chemotherapy-induced renal salt wasting was then established. Renal and central pathways were shown to be responsible for many cases of chemotherapy-induced hyponatremia. In 2005, Tho and Ferry[10] while describing a case of hyponatremia in NSCLC quite understandably raised the question of whether it is correct not to give much importance to hyponatremia in NSCLC.
In 2008, Jacot and colleagues published their results[11] of a series of 301 patients suffering from NSCLC, among whom 24 patients (8%) had pretreatment hyponatremia. Particularly notable in our small series of 40 cases of lung cancers was that only one case out of the five SCLC cases showed hyponatremia but in NSCLC an overwhelming 20% (7/35 cases) had hyponatremia, with another three cases with low-normal values who rapidly went into the hyponatremic state. In our series, hyponatremia was seen both before and during treatment, and the condition was also significantly associated with death.
The causes of such differences in the prevalence of hyponatremia between our study and data published from the West may include greater mental stress and extreme pollution of the environment prevailing in India. The high rates of hyponatremia in NSCLC may have an enormous role to play in the poor survival seen in these cases in our country, where low standards of management is generally implicated as the cause of high mortality. In any case, it is important to validate our observations in a bigger trial.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 Range of parameters in living and dead patients

| Fig. 2 Logrank test for serum sodium


 PDF
PDF  Views
Views  Share
Share

