Hypothalamic-Pituitary Axis Dysfunction and Metabolic Derangements in Thai Childhood Leukemia Survivors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(05): 688-694
DOI: DOI: 10.4103/ijmpo.ijmpo_238_19
Abstract
Aims: To determine the prevalence and severity of endocrine and metabolic derangements in childhood-onset ALL survivors from Thailand and to describe the associated risk factors. Settings and Design: Paediatric department in medical school hospital, cross-sectional study. Methods: Insulin Tolerance Test (ITT), IGF-I and IGFBP-3 levels, Thyroid and gonadal function, serum sodium and metabolic profiles were investigated in 30 childhood onset ALL survivors. (16 males, 14 females, mean age: 14.66 ± 7.16 years). Results: Endocrine abnormalities were displayed in 73.33 % of patients, 46.7% had two or more abnormalities. Grade3 of severity were present in 16.67%. Growth hormone deficiency (GHD) was detected in 10 patients (33%). Early onset of ALL was the potential risk factor of GHD. Adult height was more deteriorated in the female group. Twenty percent of patients were found with subnormal cortisol responses. Gonadal failure was evidenced in one case that experienced testicular irradiation. No diabetes insipidus was detected. Among 6 obese patients, 2 patients developed metabolic syndrome. Moreover, one patient was diagnosed with insulin-depleted diabetes mellitus. Conclusion: Our results highlighted various endocrine and metabolic sequelae occurring in childhood-onset ALL survivors after completion of their therapy. The prevalence of GHD was higher than the one previously described in Japan population. Subclinical hormonal abnormalities may affect health outcomes. Biochemical and hormonal abnormalities should be carefully monitored for immediate treatment.
Keywords
Acute leukemia - endocrine - insulin tolerance test - survivors - ThailandPublication History
Received: 28 November 2019
Accepted: 19 June 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims: To determine the prevalence and severity of endocrine and metabolic derangements in childhood-onset ALL survivors from Thailand and to describe the associated risk factors. Settings and Design: Paediatric department in medical school hospital, cross-sectional study. Methods: Insulin Tolerance Test (ITT), IGF-I and IGFBP-3 levels, Thyroid and gonadal function, serum sodium and metabolic profiles were investigated in 30 childhood onset ALL survivors. (16 males, 14 females, mean age: 14.66 ± 7.16 years). Results: Endocrine abnormalities were displayed in 73.33 % of patients, 46.7% had two or more abnormalities. Grade3 of severity were present in 16.67%. Growth hormone deficiency (GHD) was detected in 10 patients (33%). Early onset of ALL was the potential risk factor of GHD. Adult height was more deteriorated in the female group. Twenty percent of patients were found with subnormal cortisol responses. Gonadal failure was evidenced in one case that experienced testicular irradiation. No diabetes insipidus was detected. Among 6 obese patients, 2 patients developed metabolic syndrome. Moreover, one patient was diagnosed with insulin-depleted diabetes mellitus. Conclusion: Our results highlighted various endocrine and metabolic sequelae occurring in childhood-onset ALL survivors after completion of their therapy. The prevalence of GHD was higher than the one previously described in Japan population. Subclinical hormonal abnormalities may affect health outcomes. Biochemical and hormonal abnormalities should be carefully monitored for immediate treatment.
Keywords
Acute leukemia - endocrine - insulin tolerance test - survivors - ThailandIntroduction
The survival rate of acute lymphoblastic leukemia (ALL) in Thailand has improved significantly during the last decade. Notably, the 5-year overall survival of ALL in Thailand reached 67.2%.[1] Approximately 90% of childhood cancer survivors develop medical complications or deficiencies.[2] Endocrinopathies, including hypothalamic–pituitary axis (HP axis) dysfunction and metabolic derangements, represent the most frequent long-term complications associated with leukemia treatment. These endocrine abnormalities are mainly characterized by growth hormone deficiency (GHD), precocious puberty, gonadal dysfunction, metabolic syndrome, and obesity.[3],[4]
HP axis dysfunction is frequently observed in ALL survivors following chemotherapy and cranial radiotherapy (CRT).[5],[6] Growth patterns among ALL survivors commonly display height deficit and impairment of growth hormone (GH)/insulin-like growth factor (IGF-I) axis, especially in young age patients with ALL diagnosis who were treated by CRT.[7] Central hypothyroidism was rarely observed in ALL survivors. Central adrenal insufficiency (CAI) could also occur by moderate-dose CRT (18–24 Gy).[8],[9] ALL survivors treated with CRT initiated their puberty significantly earlier than those who were not treated with CRT.[10]
Posterior pituitary complication such as central diabetes insipidus (central DI), was rarely observed in ALL survivors treated with standard dose-based CRT.[3],[4]
Obesity and metabolic syndrome also affect a substantial proportion of ALL survivors with a prevalence of 30%–40%. CRT and prolonged exposure to high-dose glucocorticoids represents significant risk factors for the development of these disorders.[11]
HP axis dysfunction and metabolic derangements remain poorly investigated in Asian population, especially in Southeast Asians.[12],[13],[14],[15] Pharmacoethnicity, which refers to ethnic variance in drug response and toxicities, may affect the long-term health outcomes, together with health behavior and lifestyle differences.[16]
The goal of the present study was to measure the prevalence and severity of hormonal abnormalities and metabolic disturbances in Thai childhood ALL survivors as well as to identify the associated risk factors for prevention or better control of subsequent complications.
Materials and Methods
Selection and description of participants
Forty ALL survivors, who were in complete remission for more than 1 year, were invited to participate in this study. Thirty patients completed the full study. ALL was diagnosed when subjects were < 15 years old from 1990 to 2007. No patient treated with bone marrow transplantation was recruited into this study. Twenty-five patients (83.33%) were in the first clinical remission whereas five patients were in their second remission after treatment for a recurrence. A review of all medical records was performed.
All of the patients were treated for 2–3 years by chemotherapy including traditional drugs used for induction, consolidation and maintenance phases (vincristine, L-asparaginase, methotrexate, prednisolone, 6-mercaptopurine, etc.) according to the Thai Pediatric Oncology Group protocol.[1] Twenty-four patients (80%) received central nervous system (CNS) prophylaxis through cranial 18 Gy radiation dose. Six patients were not submitted to prophylactic CRT because their ages were under 2-year-old at the time of diagnosis; however, one underwent CRT at a later point in time for the treatment of CNS relapse. Two of the 16 male patients had testicular irradiation (18 Gy) because of testicular relapse.
This study has been approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University. (IRB approval number 18452).
Methods
All participants attended the outpatient unit from the specified time period of 7–8 a.m. Baseline data were collected. Due to the lack of standard sex and age matched childhood body mass index (BMI) curves for Thai children, we used the International Obesity Taskforce childhood BMI reference instead.[17] Patients with a BMI of more than the 85th percentile was classified as overweight; patients with a BMI of more than the 95th percentile was classified as obese. Skeletal age was determined by pediatric endocrinologists using the Greulich and Pyle method.[18] Predicted adult height (PAH) was calculated with the Bayley–Pinneau method.[19] The Final adult height (FAH) was considered achieved when annual increment was < 1 cm, with bone age of over 15 years mid-parental height (MPH) following the formula: (father's height [cm] + mother's height [cm] + 13)/2 for boys and (father's height [cm] + mother's height [cm] − 13)/2 for girls. All height was adjusted into standard deviation (SD) and SD scores (SDS), based on the Thai National Survey of Health 1997.[20]
Serum samples were collected after overnight fasting for the measurement of IGF-I, IGF binding protein-3 (IGFBP-3), thyroid-stimulating hormone (TSH), free T3, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (in female), testosterone (in male), serum sodium, fasting plasma glucose (FPG), insulin and lipid profiles (cholesterol, triglyceride [TG], high-density lipoprotein [HDL] and low-density lipoprotein).
To evaluate GH status, insulin tolerance test (ITT) was performed using blood samples collected at 0, 15, 30, 45, and 90 min. GHD was considered if GH level was lower than 5 μg/L or 10 μg/L for patients who either attained or did not attain final height, respectively.[21] The IGF-I and IGFBP-3 concentrations were measured by an automated chemiluminescent assay (IMMULITE®, Diagnostic Products Corp, Los Angeles, CA, USA).[22] IGF-I and IGFBP-3 levels were categorized into age-related −2, −1, 0, +1, +2, SD using reference ranges for normal population as reported by Elmlinger et al.[23]
HP-adrenal (HPA) axis was also evaluated during ITT. The cortisol response was considered as normal based on achievement of a peak level of 18 μg/dL or above. Patients with peak cortisol level < 4 μg/dL and 18 μg/dL were classified in the cortisol deficiency group and subnormal cortisol response group, respectively.[2]
Hypothyroidism was considered when a patient had lower free T3 and free T4 than normal range and subclinical hypothyroidism was diagnosed when a patient displayed normal free T3, free T4 and TSH level above the limit (4.1 mU/L).
Hypogonadism was characterized by failure to initiate puberty at 13 and 14-year-old for female and male subjects, respectively or by a failure to progress through puberty after the occurrence of secondary sex characteristics.
DI was screened by episodes of polyuria and polydipsia as well as high serum sodium levels.
FPG and hemoglobin A1c were assayed for diabetes mellitus (DM). DM was defined according to American Diabetes Association criteria.[24] Metabolic syndrome was defined following International Diabetes Federation clinical criteria (waist circumference ≥90th percentile, TG ≥150 mg/dL, HDL ≤40 mg/dL in male and < 50 mg/dl in female, systolic blood pressure ≥135, diastolic blood pressure ≥85 mmHg and FPG, FPG ≥100 mg/dL.).[25]
The severity of specific late complication was graded based on the Common Terminology Criteria of Adverse Events version 5.0.[26] The late effects were scored from Grades 1–5 with descriptions of severity for each adverse event (Grade 1, mild; 2, moderate; 3, severe; 4, life threatening or disabling; 5, death-related adverse event).
Statistics
Data were presented as mean ± SD or as mean (range). Mean of MPH and FAH (or PAH) was compared and analyzed using Paired-Sample t-test. Pearson's and Spearman's correlation coefficients were used to assess relationships between normally and nonnormally distributed variables, respectively. P < 0.05 was considered statistically significant. All computations were performed using the SPSS Statistics for Windows, Version 17.0. (SPSS Inc., Chicago, IL, USA).
Results
A total of 30 patients were recruited in this study, 16 male and 14 female survivors of childhood-onset ALL. Study participant baseline characteristics are shown in [Table 1]. The onset of leukemia appeared between the ages of 1.1 years and 14.1 years. Twenty-five patients (83.33%) received CRT for CNS prophylaxis. None of the patients was diagnosed with endocrine deficiency or continued hormonal therapy before their participation in this study. Endocrine abnormalities were recently detected in 22 patients (73.33%). Ten survivors (33.33%) had Grade 1 (mild) and 7 (23.33%) had Grade 2 (moderate) severity of late effects. Grade 3 was found in 16.67% of survivors.
Two patients (6.67%), one male and one female, had FAH SDS (or PAH SDS) P = 0.009). The survivors' adult height was shown not to be correlated with CRT or age at diagnosis or years of follow up after completion of treatment. Two patients with short stature (shorter than −2SD) had psychological distress for growth disorders, requiring psychiatric consultation.
Growth hormone, insulin-like growth factor -I, and insulin-like growth factor binding protein-3 axis
GHD was diagnosed in patients < 18 years old, with a prevalence reaching 45.5% (10/22 patients) [Figure 1]. The development of ALL in subjects with an age < 5-year-old was statistically correlated with peak GH and GHD (P = 0.0089, 0.033, respectively). CRT and dosage of CRT was not correlated with GH response.
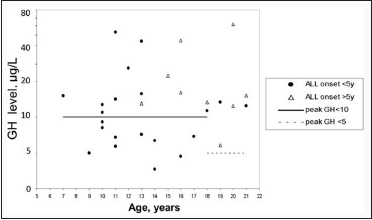
| Figure 1: Peak of Growth hormone (μg/L) in ITT
As shown in [Figure 2] and [Figure 3], IGF-I and IGFBP-3 levels reached the normal age-related reference range (−2SD to + 2SD) and the IGF-I z-score was strongly correlated to IGFBP-3 z-score (P = 0.000581). Patients diagnosed with GHD displayed lower IGF-I z-score (−0.50 ± 0.972) and IGFBP-3 z-score (−0.20 ± 1.033) compared with subjects from the non-GHD group (0.05 ± 1.146, 0.05 ± 0.887, respectively. FAH (or PAH) SDS showed no statistical correlation with GHD, IGF-I z-score and IGFBP-3 z-score.
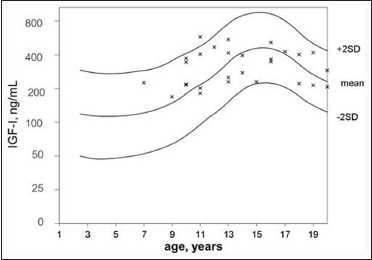
| Figure 2: Insulin-like growth factor-I (ng/mL) in age-matched reference curve
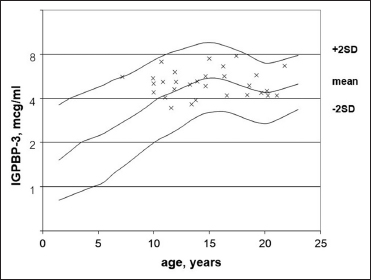
| Figure 3: Insulin-like growth factor binding protein 3 (mcg/ml) in agematched reference curve
Thyroid axis
One female with infantile ALL was diagnosed with subclinical hypothyroidism but without evidence of goiter or family history of autoimmune thyroiditis. She did not receive CRT. The thyroid dysfunction of this subject was characterized by TSH, FT4 and FT3 serum concentrations of17.73 mU/L, FT4 1.24 ng/dL and FT3 3.34 pg/mL, respectively. Antibodies to thyroglobulin and thyroperoxidase were not detected.
Gonadal function
Primary hypogonadism, low testosterone and high LH, FSH, was evidenced in one male patient who experienced two courses of chemotherapy, testicular biopsy and testicular irradiation due to testicular relapse. This subject did not show any signs of puberty at 14.8 years old.
Central precocious puberty was found in 2 of 14 female patients, both of whom underwent low dose CRT. Spontaneous puberty and menarche were observed in other female patients appropriately with their Tanner pubertal staging.
Hypothalamic-pituitary-adrenal axis
Six out of 30 subjects (20%) developed subnormal cortisol response (cortisol peak < 18 μg/dL) in ITT. No correlation was found between cortisol peak and the onset age of ALL, range of follow-up years after treatment completion or CRT. None of the patients displayed impaired HPA axis (cortisol peak <=4 μg/dL).
Diabetes insipidus
None of the participants had a history of polyuria, polydipsia or had hypernatremia suggestive of DI.
Obesity and metabolic disturbances
One female and three males out of 30 patients (16.67%) were considered obese whereas one female and one male were overweight. All of them underwent CRT for CNS prophylaxis.
One out of 30 patients was recently diagnosed with DM. He had a normal BMI together with mild symptoms of polyuria and polydipsia. The patient's blood test showed no evidence of hyperinsulinism according to the levels of fasting insulin.
Five out of 30 patients (16.66%) had hypertriglyceridemia (TG ≥ 150 mg/dL) while 10 out of 30 patients (33.33%) had reduced HDL. No patient in the study group developed systolic or diastolic hypertension [Table 2]. Two out of 30 patients (6.67%) were diagnosed with metabolic syndrome due to high TG and low HDL.
Discussion
With an average follow-up of 6 years, this study revealed that 73.33% of Thai leukemia survivors showed endocrine and metabolic derangement. The point prevalence was 33.33% for GHD, 20% for subnormal cortisol responses, 3.33% for subclinical hypothyroidism, 33.33% reduced HDL and 16.67% for obesity.
GHD was demonstrated in one-third of the patients and this prevalence was much higher than the one previously reported in Japan (2.8%),[12] but lower than the frequency measured in a large US cohort (46.5%).[27] This might be caused by the different method of GH assessment and also could be due to fewer subjects in our study. Nevertheless, the IGF-I and IGF-BP3 serum concentrations in our GHD cases were low to normal and height SDS was not compromised significantly. These data suggest that ALL treatment marginally reduced pituitary function at the level of GH production and/or secretion. Survivors with ALL onset appearing in the first 5 years of life were at risk of GHD, as described previously.[27] Therefore, continuous screening and subsequent treatment for GHD disorder should be implemented. Untreated GHD is commonly associated with abdominal obesity, low muscle mass, low energy expenditure, muscle weakness, and poor exercise tolerance over the following years.[28]
A previous study in Thailand[29] demonstrated that girls completing their ALL treatment had less adult height deficit than boys, resulting from more consistent weight gain. Conversely, in our population, we found remarkable decrease in height of female patients, which could be explained by the phenomenon of early onset puberty which is more commonly observed in female than male patients.
Abnormal thyroid function due to primary and secondary hypothyroidism has been reported after high dose CRT and even during a long period of time after treatment completion.[30] In our study, most patients received CRT at a lower dose (18 Gy) and only two received higher doses (36 and 39 Gy). However, all of them had normal thyroid function test results during our 4 year-10 month follow-up. Nevertheless, it would be interesting to screen this thyroid disorder over a longer period as central hypothyroidism could arise at any time following treatment.[31] One female with subclinical hypothyroidism of unknown origin had been treated with CRT and did not develop thyroid-specific autoantibodies. The etiology of her subclinical hypothyroidism is inconclusive.
Reproductive abnormalities could result from direct damage of the gonads by systemic chemotherapy, alteration of the HP -gonad axis and cancerous cell infiltration in the gonad.[2] One out of 16 male patients suffered from gonadal failure due to testicular relapse, a direct effect of testicular irradiation and repeated course of chemotherapy. Consequently, long-term reproductive analysis including sperm cell counting and fertility rate should be monitored in all male survivors. In female patients, 2 of 14 patients (14.28%) were diagnosed with precocious puberty. This abnormality can be associated with low-dose CRT.[32] None of the female patients from our study were reported with delayed puberty or primary amenorrhea. Nevertheless, we cannot exclude the development of other late effects such as pregnancy rate and premature menopause during a longer follow-up.
In this study, 6 subnormal cortisol responses (20% of all patients) were detected. Five of these 6 subjects were female. Although female gender seems to have poorer cortisol increment in testing, this observation remains to be explained and should be further investigated.[8],[33] Clinical signs and symptoms of adrenal insufficiency as well as a record of adrenal crisis were not evidenced in subjects with subnormal cortical response. However, glucocorticoid supplementation during major stress is considered to prevent consequences of adrenal crisis.
In agreement with previously reported studies, none of the participants suffered from DI.[34],[35] It must be pointed out that, in ALL survivors, DI does not usually result from hypothalamic and pituitary tumors because standard ALL treatment protocols are commonly based on cranial irradiation.
Some evidences suggest that cancer survivors in general and particularly survivors of childhood ALL are highly susceptible to develop metabolic syndrome.[15] Metabolic syndrome is a significant risk factor for further cardiovascular disease and DM.[36] Our present data highlighted that reduced HDL (33.33%) is the most common of metabolic disturbance which could represent the first emerging metabolic complication in childhood ALL survivors.
One patient of our cohort, without family history of DM but having mild symptoms of diabetes and no signs of insulin resistance, was newly diagnosed with DM. The measured insulin level (5.6 mU/L) and the absence of anti-GAD autoantibodies suggested that the etiology of DM for this patient resulted from pancreatic β-cell deterioration.[37]
Conclusion
The present study described the higher prevalence of subclinical abnormalities of metabolic and hormonal profiles than previously reported findings among Asian population. These disorders will negatively influence the health and quality of life of ALL survivors in the future. A systematic and periodic monitoring of growth parameters, GH axis, adrenal function and obesity is consequently recommended for all childhood-onset ALL survivors.
Conflict of Interest
There are no conflicts of interest.
Acknowledgments
This research was supported by Chula Quality Improvement Fund, King Chulalongkorn Memorial Hospital, The Thai Red Cross society.
References
- Seksarn P, Wiangnon S, Veerakul G, Chotsampancharoen T, Kanjanapongkul S, Chainansamit SO. Outcome of childhood acute lymphoblastic leukemia treated using the Thai national protocols. Asian Pac J Cancer Prev 2015; 16: 4609-14
- Rose SR, Horne VE, Howell J, Lawson SA, Rutter MM, Trotman GE. et al. Late endocrine effects of childhood cancer. Nat Rev Endocrinol 2016; 12: 319-36
- Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: Endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol 2017; 176: R183-203
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64: 83-103
- Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA. et al. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab 2004; 17: 55-66
- Gurney JG, Ness KK, Sibley SD, O'Leary M, Dengel DR, Lee JM. et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer 2006; 107: 1303-12
- Vilela MI, Serravite Mde O, Oliveira NB, de Brito PC, Ribeiro-Oliveira Jr A, Viana MB. Height deficit and impairment of the GH/IGF-1 axis in patients treated for acute lymphoblastic leukemia during childhood. Horm Res Paediatr 2013; 79: 9-16
- Follin C, Wiebe T, Moëll C, Erfurth EM. Moderate dose cranial radiotherapy causes central adrenal insufficiency in long-term survivors of childhood leukaemia. Pituitary 2014; 17: 7-12
- Gordijn MS, van Litsenburg RR, Gemke RJ, Bierings MB, Hoogerbrugge PM, van de Ven PM. et al. Hypothalamic-pituitary-adrenal axis function in survivors of childhood acute lymphoblastic leukemia and healthy controls. Psychoneuroendocrinology 2012; 37: 1448-56
- Elitzur S, Houri-Shtrecher R, Yackobovitz-Gavan M, Avrahami G, Barzilai S, Gilad G. et al. Growth and pubertal patterns in young survivors of childhood acute lymphoblastic leukemia. J Pediatr Endocrinol Metab 2017; 30: 869-77
- Zhang FF, Rodday AM, Kelly MJ, Must A, MacPherson C, Roberts SB. et al. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 2014; 61: 1263-9
- Hata M, Ogino I, Aida N, Saito K, Omura M, Kigasawa H. et al. Prophylactic cranial irradiation of acute lymphoblastic leukemia in childhood: Outcomes of late effects on pituitary function and growth in long-term survivors. Int J Cancer 2001; 96 Suppl: 117-24
- Han JW, Kwon SY, Won SC, Shin YJ, Ko JH, Lyu CJ. Comprehensive clinical follow-up of late effects in childhood cancer survivors shows the need for early and well-timed intervention. Ann Oncol 2009; 20: 1170-7
- Han JW, Kim HS, Kim BS, Kwon SY, Shin YJ, Kim SH. et al. Increasing and worsening late effects in childhood cancer survivors during follow-up. J Korean Med Sci 2013; 28: 755-62
- Zareifar S, Haghpanah S, Shorafa E, Shakibazad N, Karamizadeh Z. Evaluation of metabolic syndrome and related factors in children affected by acute lymphoblastic leukemia. Indian J Med Paediatr Oncol 2017; 38: 97-102
- Ting Cheung Y. Cancer pharmacoethnicity: Ascertaining chronic health outcomes in survivors of childhood cancer in Asia. Glob J Pharmaceu Sci 2017; 4: 555634
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000; 320: 1240-3
- Greulich WW, Pyle SI, Todd TW. Radiographic Atlas of Skeletal Development of the Hand and Wrist. California: Stanford University Press; 1950
- Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J Pediatr 1952; 40: 423-41
- National Health Foundation. Report of National Health Survey by Physical Measurement, phase II. Thailand: Ministry of Public Health; 1997
- Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M. et al. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 2005; 152: 165-70
- Babson AL, Olson DR, Palmieri T, Ross AF, Becker DM, Mulqueen PJ. The IMMULITE assay tube: A new approach to heterogeneous ligand assay. Clin Chem 1991; 37: 1521-2
- Elmlinger MW, Kühnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin Chem Lab Med 2004; 42: 654-64
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34 Suppl 1: S62-9
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S. et al. The metabolic syndrome in children and adolescents An IDF consensus report. Pediatr Diabetes 2007; 8: 299-306
- U.S. Department of Health and Human Services: National Institutes of Health and National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Available from: [last accessed on 2019 Jun 03]
- Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM. et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J Clin Oncol 2015; 33: 492-500
- Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC. et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2007; 49: 975-81
- Jaruratanasirikul S, Owasith K, Wongchanchailert M, Laosombat V, Sriplung H. Growth patterns and final height of survivors of childhood leukemia. J Pediatr Endocrinol Metab 2004; 17: 719-26
- Lando A, Holm K, Nysom K, Rasmussen AK, Feldt-Rasmussen U, Petersen JH. et al. Thyroid function in survivors of childhood acute lymphoblastic leukaemia: The significance of prophylactic cranial irradiation. Clin Endocrinol (Oxf) 2001; 55: 21-5
- Baronio F, Battisti L, Radetti G. Central hypothyroidism following chemotherapy for acute lymphoblastic leukemia. J Pediatr Endocrinol Metab 2011; 24: 903-6
- Rutter MM, Rose SR. Long-term endocrine sequelae of childhood cancer. Curr Opin Pediatr 2007; 19: 480-7
- Darzy KH, Shalet SM. Absence of adrenocorticotropin (ACTH) neurosecretory dysfunction but increased cortisol concentrations and production rates in ACTH-replete adult cancer survivors after cranial irradiation for nonpituitary brain tumors. J Clin Endocrinol Metab 2005; 90: 5217-25
- Borson-Chazot F, Brue T. Pituitary deficiency after brain radiation therapy. Ann Endocrinol (Paris) 2006; 67: 303-9
- Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer 2004; 11: 589-602
- Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL. et al. Body mass index in long-term adult survivors of childhood cancer: A report of the Childhood Cancer Survivor Study. Cancer 2005; 103: 1730-9
- Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2009; 7: 600-6
Address for correspondence
Publication History
Received: 28 November 2019
Accepted: 19 June 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Peak of Growth hormone (μg/L) in ITT

| Figure 2: Insulin-like growth factor-I (ng/mL) in age-matched reference curve

| Figure 3: Insulin-like growth factor binding protein 3 (mcg/ml) in agematched reference curve
- 1 Seksarn P, Wiangnon S, Veerakul G, Chotsampancharoen T, Kanjanapongkul S, Chainansamit SO. Outcome of childhood acute lymphoblastic leukemia treated using the Thai national protocols. Asian Pac J Cancer Prev 2015; 16: 4609-14
- 2 Rose SR, Horne VE, Howell J, Lawson SA, Rutter MM, Trotman GE. et al. Late endocrine effects of childhood cancer. Nat Rev Endocrinol 2016; 12: 319-36
- 3 Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: Endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol 2017; 176: R183-203
- 4 Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64: 83-103
- 5 Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA. et al. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab 2004; 17: 55-66
- 6 Gurney JG, Ness KK, Sibley SD, O'Leary M, Dengel DR, Lee JM. et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer 2006; 107: 1303-12
- 7 Vilela MI, Serravite Mde O, Oliveira NB, de Brito PC, Ribeiro-Oliveira Jr A, Viana MB. Height deficit and impairment of the GH/IGF-1 axis in patients treated for acute lymphoblastic leukemia during childhood. Horm Res Paediatr 2013; 79: 9-16
- 8 Follin C, Wiebe T, Moëll C, Erfurth EM. Moderate dose cranial radiotherapy causes central adrenal insufficiency in long-term survivors of childhood leukaemia. Pituitary 2014; 17: 7-12
- 9 Gordijn MS, van Litsenburg RR, Gemke RJ, Bierings MB, Hoogerbrugge PM, van de Ven PM. et al. Hypothalamic-pituitary-adrenal axis function in survivors of childhood acute lymphoblastic leukemia and healthy controls. Psychoneuroendocrinology 2012; 37: 1448-56
- 10 Elitzur S, Houri-Shtrecher R, Yackobovitz-Gavan M, Avrahami G, Barzilai S, Gilad G. et al. Growth and pubertal patterns in young survivors of childhood acute lymphoblastic leukemia. J Pediatr Endocrinol Metab 2017; 30: 869-77
- 11 Zhang FF, Rodday AM, Kelly MJ, Must A, MacPherson C, Roberts SB. et al. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 2014; 61: 1263-9
- 12 Hata M, Ogino I, Aida N, Saito K, Omura M, Kigasawa H. et al. Prophylactic cranial irradiation of acute lymphoblastic leukemia in childhood: Outcomes of late effects on pituitary function and growth in long-term survivors. Int J Cancer 2001; 96 Suppl: 117-24
- 13 Han JW, Kwon SY, Won SC, Shin YJ, Ko JH, Lyu CJ. Comprehensive clinical follow-up of late effects in childhood cancer survivors shows the need for early and well-timed intervention. Ann Oncol 2009; 20: 1170-7
- 14 Han JW, Kim HS, Kim BS, Kwon SY, Shin YJ, Kim SH. et al. Increasing and worsening late effects in childhood cancer survivors during follow-up. J Korean Med Sci 2013; 28: 755-62
- 15 Zareifar S, Haghpanah S, Shorafa E, Shakibazad N, Karamizadeh Z. Evaluation of metabolic syndrome and related factors in children affected by acute lymphoblastic leukemia. Indian J Med Paediatr Oncol 2017; 38: 97-102
- 16 Ting Cheung Y. Cancer pharmacoethnicity: Ascertaining chronic health outcomes in survivors of childhood cancer in Asia. Glob J Pharmaceu Sci 2017; 4: 555634
- 17 Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000; 320: 1240-3
- 18 Greulich WW, Pyle SI, Todd TW. Radiographic Atlas of Skeletal Development of the Hand and Wrist. California: Stanford University Press; 1950
- 19 Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J Pediatr 1952; 40: 423-41
- 20 National Health Foundation. Report of National Health Survey by Physical Measurement, phase II. Thailand: Ministry of Public Health; 1997
- 21 Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M. et al. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 2005; 152: 165-70
- 22 Babson AL, Olson DR, Palmieri T, Ross AF, Becker DM, Mulqueen PJ. The IMMULITE assay tube: A new approach to heterogeneous ligand assay. Clin Chem 1991; 37: 1521-2
- 23 Elmlinger MW, Kühnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin Chem Lab Med 2004; 42: 654-64
- 24 American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34 Suppl 1: S62-9
- 25 Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S. et al. The metabolic syndrome in children and adolescents An IDF consensus report. Pediatr Diabetes 2007; 8: 299-306
- 26 U.S. Department of Health and Human Services: National Institutes of Health and National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Available from: [last accessed on 2019 Jun 03]
- 27 Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM. et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J Clin Oncol 2015; 33: 492-500
- 28 Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC. et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2007; 49: 975-81
- 29 Jaruratanasirikul S, Owasith K, Wongchanchailert M, Laosombat V, Sriplung H. Growth patterns and final height of survivors of childhood leukemia. J Pediatr Endocrinol Metab 2004; 17: 719-26
- 30 Lando A, Holm K, Nysom K, Rasmussen AK, Feldt-Rasmussen U, Petersen JH. et al. Thyroid function in survivors of childhood acute lymphoblastic leukaemia: The significance of prophylactic cranial irradiation. Clin Endocrinol (Oxf) 2001; 55: 21-5
- 31 Baronio F, Battisti L, Radetti G. Central hypothyroidism following chemotherapy for acute lymphoblastic leukemia. J Pediatr Endocrinol Metab 2011; 24: 903-6
- 32 Rutter MM, Rose SR. Long-term endocrine sequelae of childhood cancer. Curr Opin Pediatr 2007; 19: 480-7
- 33 Darzy KH, Shalet SM. Absence of adrenocorticotropin (ACTH) neurosecretory dysfunction but increased cortisol concentrations and production rates in ACTH-replete adult cancer survivors after cranial irradiation for nonpituitary brain tumors. J Clin Endocrinol Metab 2005; 90: 5217-25
- 34 Borson-Chazot F, Brue T. Pituitary deficiency after brain radiation therapy. Ann Endocrinol (Paris) 2006; 67: 303-9
- 35 Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer 2004; 11: 589-602
- 36 Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL. et al. Body mass index in long-term adult survivors of childhood cancer: A report of the Childhood Cancer Survivor Study. Cancer 2005; 103: 1730-9
- 37 Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2009; 7: 600-6


 PDF
PDF  Views
Views  Share
Share

