Imaging Recommendations for Diagnosis, Staging, and Management of Cancer of the Thyroid, Parathyroid, and Salivary Glands
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 159-174
DOI: DOI: 10.1055/s-0042-1760403
Abstract
Thyroid cancer ranks as the leading endocrine malignancy in adults. The foundation for primary diagnosis of thyroid cancer is a high-resolution ultrasound (US) of the thyroid gland including US-guided fine-needle biopsy (FNB) of suspected thyroid nodules. Advanced cross-sectional imaging, including computed tomography (CT), magnetic resonance imaging, and positron emission tomography, can be useful in selected patients. The mainstay of treatment of thyroid cancer is surgery. It may be supplemented by radioactive iodine ablation/therapy in high-risk differentiated thyroid cancer. Radiology plays a crucial role in both diagnostic and posttreatment follow-up imaging. Primary hyperparathyroidism (PHPT) is the third most common endocrine disorder with single parathyroid adenoma being its most common cause. The radiologist's aim in parathyroid imaging is to provide the clinician with an illustrative picture of the neck, locating lesions with respect to landmarks. Imaging helps in the detection of solitary versus multiglandular disease, ectopic and supernumerary glands with precise localization. US, nuclear imaging, and four-dimensional CT are the most commonly used imaging modalities for the preoperative localization of the parathyroid disease. Salivary gland tumors account for approximately 0.5%-of all neoplasms, the most common location being the parotid gland (70%). Imaging is crucial in salivary gland tumors by defining its location, detecting malignant features, assessing local extension and invasion, staging the tumors according to the tumor-node-metastasis classification, and assessing the feasibility of surgery.
Keywords
Computed tomography - cross-sectional imaging - guidelines - head and neck cancer - magnetic resonance imaging - parathyroid imaging - salivary gland tumor - thyroid cancer - thyroid gland - ultrasound - thyroid gland - ultrasoundPublication History
Article published online:
04 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Thyroid cancer ranks as the leading endocrine malignancy in adults. The foundation for primary diagnosis of thyroid cancer is a high-resolution ultrasound (US) of the thyroid gland including US-guided fine-needle biopsy (FNB) of suspected thyroid nodules. Advanced cross-sectional imaging, including computed tomography (CT), magnetic resonance imaging, and positron emission tomography, can be useful in selected patients. The mainstay of treatment of thyroid cancer is surgery. It may be supplemented by radioactive iodine ablation/therapy in high-risk differentiated thyroid cancer. Radiology plays a crucial role in both diagnostic and posttreatment follow-up imaging. Primary hyperparathyroidism (PHPT) is the third most common endocrine disorder with single parathyroid adenoma being its most common cause. The radiologist's aim in parathyroid imaging is to provide the clinician with an illustrative picture of the neck, locating lesions with respect to landmarks. Imaging helps in the detection of solitary versus multiglandular disease, ectopic and supernumerary glands with precise localization. US, nuclear imaging, and four-dimensional CT are the most commonly used imaging modalities for the preoperative localization of the parathyroid disease. Salivary gland tumors account for approximately 0.5%-of all neoplasms, the most common location being the parotid gland (70%). Imaging is crucial in salivary gland tumors by defining its location, detecting malignant features, assessing local extension and invasion, staging the tumors according to the tumor-node-metastasis classification, and assessing the feasibility of surgery.
Keywords
Computed tomography - cross-sectional imaging - guidelines - head and neck cancer - magnetic resonance imaging - parathyroid imaging - salivary gland tumor - thyroid cancer - thyroid gland - ultrasound - thyroid gland - ultrasoundThyroid Gland, Introduction, Risk Factors, and Etiopathogenesis
Thyroid cancer ranks as the leading endocrine malignancy in adults.[1] Thyroid cancer can arise from two chief cell types: follicular and parafollicular or C-cells. Papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) are grouped together as differentiated thyroid cancer (DTC) as they both arise from thyroid follicular cells. Medullary thyroid cancer arises from parafollicular cells. Anaplastic carcinomas (ATC) are the most undifferentiated type of thyroid cancer.[2] Papillary microcarcinoma (PMC) refers to papillary cancer smaller than 1 cm.[3]
Exposure to radiation, family history of thyroid cancer, and inherited genetic syndromes are some of the risk factors for thyroid cancer. Other risk factors include high iodine intake, increased thyroid-stimulating hormone (TSH) levels, obesity, and exposure to nitrate.[4] Sources of radiation exposure may be environmental nuclear disasters, radiological tests (e.g., computed tomography [CT] scans, X-rays), and medical treatment, including radiation therapy for head and neck cancer and radioactive iodine (RAI) treatment.[5] Thyroid cancer is known to have a high hereditary predisposition; however, more than 90%-is sporadic in nature.[6] [7] The autosomal dominant hereditary syndromes associated with thyroid cancer include familial adenomatous polyposis, Cowden syndrome, and Carney complex. The familial form of medullary thyroid carcinoma (MTC) is present in 20 to 25%-of cases and is usually a component of MEN 2A or 2B or a part of familial medullary thyroid carcinoma syndrome.[8]
Epidemiology, Clinical Presentation in India and Global
The GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer show that there were 586,202 new cases of thyroid cancer with an estimated 43,646 deaths worldwide with an estimated incidence rate of 1.4 per 100,000 in India.[9] DTC accounts for 90%-of all cases with PTC accounting for approximately 70 to 80%-and FTC accounting for 10 to 15%-of all cases.[10] PMC accounts for approximately 24%-of thyroid cancer cases in the United States.[11] Most cases of DTC occur in adults ages 30 to 50 years.[12] The DTCs usually present as thyroid nodules which can be single or multiple. These cancers are more common in females. The findings which should alert the physician to thoroughly investigate the patients include extremes of age (<16>55 years), male patients, history of radiation exposure, family history, and symptoms of involvement of surrounding structures like hoarseness, breathing difficulty, and hemoptysis.
Imaging Referral Guidelines
The 2015 American Thyroid Association (ATA) management guidelines for adult patients with thyroid nodules are widely popular for DTC and MTC. The ATA guidelines for management of patients with ATC have been revised in 2021. Korean Society of Thyroid Radiology (KSThR) through a collaboration with the National Evidence-based Healthcare Collaborating Agency has developed the 2020 Imaging guidelines for thyroid nodules and DTC using an adaptation process and has identified four clinical situations which mandate thyroid imaging.[12] They reviewed the previous existing guidelines including the 2015 ATA management guidelines for adult patients with thyroid nodules and DTC, American Association of Clinical Endocrinologists (AACE)/Italian Association of Clinical Endocrinologists/the European Thyroid Association medical guidelines for clinical practice for diagnosis and management of thyroid nodules, British Thyroid Association guidelines for the management of thyroid cancer, U.S. Diagnosis and Imaging-based Management of Thyroid Nodules: Revised KSThR Consensus Statement and Recommendations, American College of Radiology (ACR) Appropriateness Criteria Thyroid Disease, Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer and National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Thyroid Carcinoma, Version 1. 2019.[13] [14] [15] [16] [17] [18] After a thorough discussion and comparison of the recommendations and the evidence in the literature for the selected guidelines, recommendations were prepared by the guideline committee.
The recommendations in four typical clinical situations include (1) evaluation of known or suspected thyroid nodules—neck US is usually appropriate for the evaluation of suspected thyroid nodules. US-guided biopsy of thyroid nodules is also appropriate for initial diagnostic workup. Contrast-enhanced CT or magnetic resonance imaging (MRI) of the neck can be additionally performed in advanced thyroid cancer. (2) Preoperative evaluation of DTC—neck US is usually appropriate for the preoperative evaluation of DTC. For the preoperative assessment of DTC, washout thyroglobulin (Tg) for neck lymph nodes (LNs) and/or ultrasound (US) guided biopsy are typically appropriate. In advanced disease, neck CT or MRI with IV contrast may be appropriate as an adjunct to US. Contrast-enhanced CT (CECT) chest may be appropriate to evaluate the lung parenchyma or the advanced mediastinum disease. (3) Early imaging after surgery for DTC—neck US is appropriate for the postoperative evaluation of DTC after definitive treatment. CECT neck may be appropriate for early imaging after definitive treatment of DTC. When the response to therapy cannot be adequately assessed by US and serum Tg, it may be taken into consideration in individuals with a high risk of persistent pathology. (4) Suspected recurrence of DTC—neck US is usually appropriate when recurrence of DTC is suspected. US-guided biopsy and/or washout Tg for neck LNs are usually appropriate when recurrence of DTC is suspected on imaging. Contrast-enhanced neck CT or MRI may be appropriate as a second-line imaging modality in cases of widely distributed recurrent disease, when aerodigestive tract invasion is suspected, or in cases of discrepancy between serum Tg and US results. Chest CT with or without IV contrast may be appropriate in high-risk patients with elevated serum Tg or rising anti-Tg antibodies.[12]
2015 ATA guidelines for MTC: US examination of the neck should be performed in all patients with MTC. Patients with significant neck disease and signs or symptoms of local or distant metastases might as well have CECT of the neck and chest, three-phase contrast-enhanced multidetector liver imaging, contrast-enhanced MRI of the liver, axial MRI, and bone scintigraphy. These studies should also be conducted in all patients with a serum Ctn level greater than 500 pe/mL. (C)[19]
2021 ATA guidelines for ATC: Initial radiological tumor staging should include cross-sectional imaging, in particular, CT neck, chest, abdomen, and pelvis with contrast (or MRI), and, if available, fluorodeoxyglucose positron emission tomography (FDG PET)/CT. If clinically necessary, contrast-enhanced brain imaging using an MRI should also be performed.[20]
Clinical/Diagnostic Work-Up Excluding Imaging
The physical examination with neck palpation and US are the two techniques to screen for thyroid cancer. Neck palpation can identify palpable nodules, while US can identify both palpable and nonpalpable nodules. The clinical examination comprises meticulous palpation of the gland along with the neck nodes. Signs of extrathyroidal extension like the involvement of straps and retrosternal extension should be assessed. Determination of Tg in the case of DTC and calcitonin in the case of MTC is of vital significance.[21] The incidence of malignancy in this scenario is low. The ATA guidelines for the assessment of thyroid nodules recommend serum TSH measurement. DTC is more commonly associated with the euthyroid or hypothyroid state. If the TSH is below normal limits, thyroid scintigraphy should be done. Focal FDG uptake in >1 cm thyroid nodule raises concern and fine-needle aspiration (FNA) should be performed. If it measures <1 href="https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1760403#JR221750877-22" xss=removed>22] The 2015 ATA guidelines for MTC suggest that family history should be thoroughly elicited in suspected medullary thyroid cancers as 25%-are hereditary. Fine-needle aspiration cytology (FNAC) from the thyroid nodule establishes the diagnosis in corroboration with serum calcitonin and CEA levels. Genetic testing and counseling should be performed to identify the RET germline mutation. The extent of the disease is established on imaging along with serum calcitonin levels. The family should be screened for the mutation and if present then prophylactic thyroidectomy should be offered to these patients.[23] [24] Somatic alterations of BRAF are common in ATC with an incidence of 40 to 70%. Immunohistochemical detection of the most common BRAF mutation (BRAFV600E) is necessary novel mutation-specific therapies that are now available.[20]
Imaging Guidelines
Diagnosis (Including Interventions)
Imaging Thyroid Nodule
The foundation for primary diagnosis of thyroid cancer is a high-resolution US of the thyroid gland including US-guided FNB of suspected thyroid nodules.[4] The US scanning technique involves the use of a high-frequency linear array probe. The scan is performed in the hyperextended position of the neck. The neck is scanned from the submental area to the sternal notch. The evaluation of the primary thyroid lesion is done along with the assessment of lateral and central compartment lymph nodes. The head is turned away from the side of interest to scan the tracheoesophageal groove. The transducer is angled inferiorly to examine the mediastinum and laterally to study lateral compartment lymph nodes (levels 2, 3, and 4). Neck is studied from mandible to clavicle and the transducer is angled inferiorly towards clavicle to image infraclavicular nodes at the base of level 4. Sweep laterally along the clavicle to posterior border of the sternocleidomastoid muscle, then trace posterior border superiorly to the mastoid process to image the posterior compartment nodes (level 5).
There are five criteria to identify malignancy on brightness-mode US that help identify malignancy in thyroid nodules: composition, hypoechogenicity, taller than wide shape (anterior-posterior diameter larger than the width in an axial scan), irregular margin, and macro- and microcalcification.[25] Many simple classification systems have been lately developed for thyroid nodules on thyroid US. The most popular include the classifications proposed by the ATA[26] and AACE,[27] and the thyroid imaging reporting and data systems (TIRADS) released by the KSThR,[28] the European Thyroid Association,[29] and the American College of Radiology (ACR-TIRADS).[30] [Table 1] describes the US features of high, intermediate, and mild suspicion of malignancy according to ATA guidelines. [Table 2] describes the ACR-TIRADS US-based thyroid nodule reporting system (discussed in [Table 2]). The performance of the TIRADS was compared in a recent meta-analysis, wherein categories 4 and 5 had a sensitivity of approximately 90%-for the detection of DTC with specificities between 50 and 60%-and ACR-TIRADS was found to be the most specific system in a recent prospective study.[31] From a size of 10 mm and above, every suspicious thyroid nodule should undergo US-guided FNB.[13] ATA and AACE guidelines recommend performing thyroid scintigraphy in addition to ultrasound in all patients evaluated for thyroid nodules who have subnormal serum thyrotropin (TSH).[13] [27]
|
Risk of malignancy |
Ultrasound features |
|---|---|
|
Benign pattern (0%-risk) |
Completely cystic nodules with well-defined walls |
|
Very low suspicion pattern (<3> |
Spongiform nodules and nodules with interspersed cystic spaces, without any of the features in more suspicious patterns |
|
Low suspicion pattern (5–10%- risk) |
Isoechoic or hyperechoic nodule partially cystic nodule with a peripheral solid component none of the following features: microcalcifications (see other points below*) irregular margins extrathyroidal extension taller than wide |
|
Intermediate suspicion pattern (10–20%-risk) |
Hypoechoic solid nodule with smooth margins none of the following features: microcalcifications (see other points below*) irregular margins extrathyroidal extension taller than wide |
|
High suspicion pattern (>70–90%- risk) |
Solid hypoechoic nodule (or solid hypoechoic component of a partially cystic nodule), with at least one of these features: microcalcifications (see other points below*) irregular margins (infiltrative, microlobulated) extrathyroidal extension taller than wide rim calcifications with an extrusive soft tissue component lymphadenopathy * Other points: - Dystrophic calcifications (e.g. coarse macrocalcification, rim calcifications) other than microcalcifications increase risk, but to a lesser degree than microcalcifications. - The cervical lymph nodes must be studied in all neck ultrasounds. |
Category |
Ultrasound finding |
Points |
|---|---|---|
|
Composition (choose one) |
Cystic or completely cystic |
0 |
|
Spongiform |
0 |
|
|
Mixed cystic and solid |
1 |
|
|
Solid or almost completely solid |
2 |
|
|
Echogenicity (choose one) |
Anechoic |
0 |
|
Hyper- or isoechoic |
1 |
|
|
Hypoechoic |
2 |
|
|
Very hypoechoic |
3 |
|
|
Shape (assessed on the transverse plane; choose one) |
Wider than tall |
0 |
|
Taller than wide |
3 |
|
|
Margin (choose one) |
Smooth |
0 |
|
Ill-defined |
0 |
|
|
Lobulated/irregular |
2 |
|
|
Extra-thyroidal Extension |
3 |
|
|
Echogenic foci (choose one or more) |
None |
0 |
|
Large comet-tail artifact |
0 |
|
|
Macrocalcifications |
1 |
|
|
Peripheral/rim calcifications |
2 |
|
|
Punctate echogenic foci |
3 |
|
Tx |
Primary tumor cannot be assessed |
|---|---|
|
T0 |
No evidence of primary tumor is found |
|
T1 |
Tumor size ≤ 2 cm in greatest dimension and is limited to the thyroid |
|
T1 a |
Tumor ≤ 1 cm, limited to the thyroid |
|
T1 b |
Tumor > 1 cm but ≤ 2 cm in greatest dimension, limited to the thyroid |
|
T2 |
Tumor size > 2 cm but ≤ 4 cm, limited to the thyroid |
|
T3 |
Tumor size > 4 cm, limited to the thyroid or any tumor with gross extrathyroidal extension invading only strap muscles |
|
T3 a |
Tumor size > 4 cm, limited to the thyroid |
|
T3 b |
Any size tumor with gross extrathyroidal extension invading only strap muscles (e.g., extension to sternothyroid, sternohyoid, thyrohyoid, or omohyoid muscles) |
|
T4 a |
Any size tumor with gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
|
T4 b |
Any size tumor with gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels |
|
NX |
Regional nodes cannot be assessed |
|---|---|
|
N0 |
No regional lymph node metastasis |
|
N0a |
One or more cytologically or histologically confirmed benign lymph nodes |
|
N0b |
No radiologic or clinical evidence of locoregional lymph node metastasis |
|
N1 |
Regional lymph node metastasis |
|
N1a |
Metastases to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian or upper mediastinal) lymph nodes; can be unilateral or bilateral disease |
|
N1b |
Metastases to unilateral, bilateral, or contralateral neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes |
|
NX |
Regional nodes cannot be assessed |
|
M0 |
No distant metastasis is found |
|
M1 |
Distant metastasis is present |
|
Stage |
T |
N |
M |
|---|---|---|---|
|
Differentiated thyroid cancer (DTC) |
|||
|
If age at diagnosis <55> |
|||
|
I |
Any T |
Any N |
M0 |
|
II |
Any T |
Any N |
M1 |
|
If age at diagnosis ≥55 y: |
|||
|
I |
T1, T2 |
N0, Nx |
M0 |
|
II |
T1, T2 |
N1 |
M0 |
|
T3 |
Any N |
M0 |
|
|
III |
T4a |
Any N |
M0 |
|
IV A |
T4b |
Any N |
M0 |
|
IV B |
Any T |
Any N |
M1 |
|
Anaplastic thyroid cancer (ATC) |
|||
|
IV A |
T1, T2, T3a |
N0, Nx |
M0 |
|
IV B |
T1, T2, T3a |
N1 |
M0 |
|
T3b, T4 |
Any N |
M0 |
|
|
IV C |
Any T |
Any M |
M1 |
|
Medullary thyroid cancer (MTC) |
|||
|
I |
T1 |
N0 |
M0 |
|
II |
T2, T3 |
N0 |
M0 |
|
III |
T1, T2, T3 |
N1 a |
M0 |
|
IV A |
T1, T2, T3 |
N1 b |
M0 |
|
T4a |
Any N |
M0 |
|
|
IV B |
T4b |
Any N |
M0 |
|
IV C |
Any T |
Any N |
M1 |
|
Protocol features |
Parameters |
|---|---|
|
Coverage |
Maxilla to carina |
|
Iodinated contrast material administration |
100 mL (370 mg iodine/mL) injected at 4 mL/s, followed by 40 mL saline flush |
|
Phases |
Noncontrast, arterial, venous |
|
Arterial phase |
30 s after start of injection |
|
Venous phase |
60 s after start of injection |
|
Thickness |
1.25 mm |
|
Tube voltage (kVp) |
140 |
|
Tube current (mA) |
Minimum 180 and maximum 300 |
|
Noise index |
10 |
|
Pitch |
1.375 |
|
DFOV (cm) |
25 |
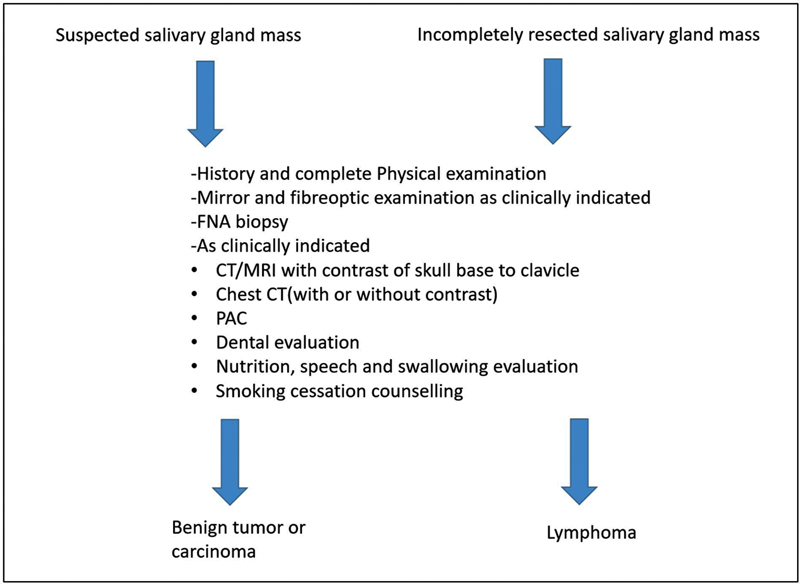
| Figure 1:Imaging recommendations by NCCN guidelines.
Imaging recommendations by American Society of Clinical Oncology (ASCO) guidelines[94]:
Imaging with neck US, CT with intravenous contrast, and/or MRI of the neck and primary site should be performed in patients with a suspicion of a salivary gland tumor.
US may be used for the initial evaluation of a new mass in adult patients, differentiating extra from intraglandular masses and identifying features that are suspicious for malignancy.
When there is a concern for malignancy such as neck adenopathy or cranial nerve dysfunction or full tumor delineation is required for operative planning, CE-MRI of the glands and neck is recommended particularly when there is a concern for skull base invasion and/or perineural tumor spread along the large named nerves.
In patients with suspicion of salivary gland cancer and involvement of adjacent bone, CT of the neck with IV contrast should be performed.
CE-MRI with a diffusion sequence of the neck and skull base is recommended for patients with suspicion of salivary gland cancer with concern for perineural invasion and/or skull base involvement.
PET/CT from the skull base to mid-thighs may be performed for patients with advanced-stage high-grade salivary gland cancers. Although there is no literature to support the use of 18 F-FDG PET/CT for initial evaluation as it has poor spatial resolution, it most accurately predicts the extent of nodal and distant metastasis disease and identifies locoregional recurrence.[95]
Imaging Recommendations for Diagnosis, Staging, and Management of Salivary Gland Tumors
Imaging plays a pivotal role in masses of salivary gland origin by defining its location, detecting malignant features, assessing local extension and invasion, staging the tumors according to the TNM classification, and also to assess the feasibility of surgery, being the primary treatment of most salivary gland tumors. It defines the location of the salivary gland tumors, whether it is intra- or extraglandular or is superficial or deep to the facial nerve, laterality (unilateral or bilateral), focality (unifocal or multifocal), characteristics of the tumor, the presence of perineural spread (PNS), and detects nodal metastasis and distant/systemic involvement. High-resolution US is the preferred initial imaging modality for lesions involving the superficial part of the parotid, submandibular, and sublingual gland as it provides excellent resolution and characterization of lesions as well as cervical node involvement without a radiation hazard. However, for all tumors detected in the sublingual gland, an MRI should be performed as the risk of malignancy is high. It is readily combined with FNAC. On the contrary, MRI is indicated in all patients with lesions of the deep lobe of parotid gland, minor salivary gland tumors, and a malignant tumor is suspected clinically.
Imaging in Diagnosis
Ultrasound
High-resolution US using a probe of frequency of 5 to 12 MHz with the Doppler technique plays an important role in the initial diagnostic workup for superficial parotid and submandibular swelling. It should be done bilaterally in paired glands in the same session. Benign tumors of the salivary gland are more common than the malignant lesions (3%).[96] US helps in the diagnosis of benign tumors by characterizing the size, echogenicity, margins, and vascularity of the lesion. US is also an optimal tool to guide fine-needle aspiration cytology with its easy availability and ability to provide real-time image guidance and has two salient roles namely, diagnosis of primary and staging of lymph node metastases. The pitfall of US in diagnosis is its limited visualization of the deep lobe of the parotid gland.[97] The minor salivary glands are also located in the mucosa of the oral cavity, pharynx, and tracheobronchial tree, but these areas are inaccessible for the evaluation by US. Additionally, as the first station, nodal drainage from the oral cavity and pharyngeal mucosal space is retropharyngeal nodes, it is also not accessible by conventional US. High variability of results has been reported for US studies, with sensitivity ranging from 62 to 84%, specificity from 88 to 96%, and accuracy from 57 to 96%,[19] with the plausible reason being that US is an operator-dependent modality.
Magnetic Resonance Imaging
MRI remains the favored imaging modality for staging and is indicated in all patients in whom a malignant tumor is suspected clinically because of its invaluable soft tissue contrast and multiplanar representation, detecting deep tissue extension, marrow infiltration, and importantly PNS and localizing parotid portion of facial nerve using high-resolution techniques. Routine locoregional tumor mapping is based on high-resolution multiplanar turbo spin-echo (TSE) T1, T2, and postcontrast (gadolinium) images with fat saturation (FS). With the administration of contrast, depiction of subtle tumor extension is possible, however, the main function is in the assessment of invasion into adjacent structures and demonstration of abnormal nodular enhancement along the facial or trigeminal nerves suggestive of PNS.[98] Typical imaging characteristics of high-grade malignant salivary gland tumors are ill-defined borders, low T2 SI, adjacent tissue or compartment invasion, heterogeneous enhancement, central necrosis, cystic changes, and bone erosion, whereas low-grade malignant tumors may resemble benign lesions showing well-defined borders, circumscribed margins, high T2 SI, and homogenous postcontrast enhancement.[99] Lymph node metastases are a feature of malignant tumors. DWI is also used for the differentiation of malignant from benign tumors as high-grade malignant tumors show an apparent diffusion coefficient value between 0.79 and 1.10, 103 /mm2/s, which is significantly lower than that of benign tumors.[100] Time-intensity curves are plotted with the help of dynamic contrast-enhanced MR, showing four different patterns: type A—persistent pattern (T-peak of >120 s without washout ratio—WR) representing benign disease, type B—plateau pattern (T-peak of >120 seconds with > 30%-WR) representing Warthin's tumors, type C—washout pattern (T-peak of <120 href="https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1760403#JR221750877-101" xss=removed>101]
Contrast-Enhanced Computed Tomography
Indications of performing CECT are a contraindication to MRI (claustrophobia, cardiac pacemaker, or ferro metallic prosthesis) or as an adjunct to MRI with suspicious bone erosion. Thin slices are acquired (0.6–1.0 mm) with both high kernel bone and soft tissue algorithms and multiplanar reconstruction.
Fluorodeoxyglucose Positron Emission Tomography Computed Tomography
The role of FDG PET/CT in the initial staging of salivary gland tumors is currently controversial; however, early detection of distant metastasis is one of its advantages. Distant metastases are common at presentation with high-grade malignant tumors as compared with low grade and also they show low avidity for FDG PET CT is not routinely recommended for the evaluation of low-grade malignant tumors.[102]
Imaging During Follow-Up
Clinical examination becomes onerous after curative treatment due to altered anatomy and fibrosis. Seventy percent of recurrences of high-grade malignant tumors are within three years; hence, follow-up periods are reported from 3 to 10 years or lifelong.[103] [104] The most common sites of distant metastasis are located within the lung followed by bones, liver, and brain.
- US: It is a rapid, easily available modality to differentiate solid from cystic lesions in the superficial areas of the head and neck, look for its vascularity, and to rule out nodal recurrence. Additionally, US-guided FNAC helps in establishing the diagnosis of recurrent disease or nonmalignant complications.
- MRI: MRI with DWI and DCE-MRI helps in the recognition of early recurrent disease during follow-up. It is recommended to have a baseline study at 3 months after the completion of therapy that serves as an invaluable road map and increases the level of confidence to demonstrate or exclude recurrent disease.[105] As per the ASCO consensus guidelines, serial MRI should be performed at 6 to 12 months for initial 2 years in cases where the probability of locoregional recurrence is high including high-grade tumors and locally advanced tumors. Subsequent to it, imaging should be performed in accordance with the symptoms and clinical examination findings. After the completion of 5 years, chest CT may be performed to detect distant metastasis in cases with high-risk histopathology features.
- FDG PET/CT: Recent papers do not demonstrate a significant contribution during surveillance due to a lot of false-positive and false-negative results.[106]
Principles of Management
Surgery is the mainstay of treatment and should be offered to all patients with a resectable locoregional disease without distant metastasis. The surgery for the primary thyroid disease is determined by the extent of local disease. Superficial parotidectomy is done for small lesions confined to the superficial lobe, total conservative parotidectomy for tumors reaching or involving the deep lobe, T3/4 or high-grade tumors and radical or extended radical parotidectomy for extensive disease involving the surrounding structures. Every attempt should be made to preserve the functional facial nerve which should only be sacrificed if grossly infiltrated or involved by the disease. Elective neck dissection clearing level II–IV should be performed for high-grade tumors and locally advanced T3/4 lesions. Therapeutic neck dissection clearing level I–V should be done in cases with regional metastasis. Adjuvant radiotherapy should be administered based on the high-risk features on histopathology like high grade, T3/4 disease, regional metastasis, perineural invasion, lymphovascular emboli, etc. Any locoregional recurrence if completely resectable should be excised and adjuvant radiotherapy if feasible should be offered. Even if the patients develop distant metastasis locoregional disease clearance should be considered especially in low-grade tumors if the metastasis is not rapidly progressing or endangering life. Radiotherapy should be recommended for unresectable tumors. The role of upfront or adjuvant chemotherapy is not established and hence such an approach should be considered mainly in a trial setting (ref ASCO consensus guidelines).
Conventional follow-up surveillance regimens include a detailed history and clinical examination every 1 to 3 months for the 1st year after completion of treatment, every 2 to 6 months in the second year, and every 4 to 8 months for years 3 to 5. When 5 years have elapsed after the completion of treatment without evidence of recurrence, the patient may follow up on an annual basis. Reimaging of the primary site and neck may be considered for anatomic areas difficult to visualize or palpate on clinical examination. MRI is the modality of choice for reimaging. PET/CT has limited utility in initial staging and has little utility in routine posttreatment surveillance. Chest imaging with CT with or without contrast should be obtained for surveillance imaging in ACC, due to the propensity for delayed distant metastatic disease.
Conflict Of Interest
None declared.
Supplementary Material
References
- Tufano RP, Clayman G, Heller KS. et al; American Thyroid Association Surgical Affairs Committee Writing Task Force. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid 2015; 25 (01) 15-27
- Noone AM, Cronin KA, Altekruse SF. et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomarkers Prev 2017; 26 (04) 632-641
- John AM, Jacob PM, Oommen R, Nair S, Nair A, Rajaratnam S. Our experience with papillary thyroid microcancer. Indian J Endocrinol Metab 2014; 18 (03) 410-413
- Liu Y, Su L, Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol 2017; 2017: 5308635
- Crnčić TB, Tomaš MI, Girotto N, Ivanković SG. Risk factors for thyroid cancer: what do we know so far?. Acta Clin Croat 2020; 59 (Suppl. 01) 66-72
- Bonnefond S, Davies TF. Thyroid cancer-risks and causes. Oncol Hematol Rev 2014; 10 (02) 14451
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013; 13 (03) 184-199
- Patel KN, Yip L, Lubitz CC. et al. The American Association of Endocrine Surgeons Guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg 2020; 271 (03) e21-e93
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- O'Neill CJ, Oucharek J, Learoyd D, Sidhu SB. Standard and emerging therapies for metastatic differentiated thyroid cancer. Oncologist 2010; 15 (02) 146-156
- Lechner MG, Hershman JM. Thyroid Nodules and Cancer in the Elderly. In: Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. PMID: 25905203.
- Lee JY, Baek JH, Ha EJ. et al; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2020 imaging guidelines for thyroid nodules and differentiated thyroid cancer: Korean Society of Thyroid Radiology. Korean J Radiol 2021; 22 (05) 840-860
- Haugen BR, Alexander EK, Bible KC. et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26 (01) 1-133
- Gharib H, Papini E, Paschke R. et al; AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive Summary of recommendations. J Endocrinol Invest 2010; 33 (5, Suppl): 287-291
- Perros P, Boelaert K, Colley S. et al; British Thyroid Association. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014; 81 (Suppl. 01) 1-122
- Shin JH, Baek JH, Chung J. et al; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2016; 17 (03) 370-395
- Yi KH, Park YJ, Koong SS. et al. Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Endocrinol Metab (Seoul) 2010; 25 (04) 270-297
- www.nccn.org
- Wells Jr SAJ, Asa SL, Dralle H. et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25 (06) 567-610
- Bible KC, Kebebew E, Brierley J. et al. 2021 American thyroid association guidelines for management of patients with anaplastic thyroid cancer: American thyroid association anaplastic thyroid cancer guidelines task force. Thyroid 2021; 31 (03) 337-386
- Seib CD, Harari A, Conte FA, Duh QY, Clark OH, Gosnell JE. Utility of serum thyroglobulin measurements after prophylactic thyroidectomy in patients with hereditary medullary thyroid cancer. Surgery 2014; 156 (02) 394-398
- Cibas ES, Ali SZ. The 2017 Bethesda System for reporting thyroid cytopathology. Thyroid 2017; 27 (11) 1341-1346
- Birtwhistle R, Morissette K, Dickinson JA. et al; Canadian Task Force on Preventive Health Care. Recommendation on screening adults for asymptomatic thyroid dysfunction in primary care. CMAJ 2019; 191 (46) E1274-E1280
- Morrison SA, Suh H, Hodin RA. The surgical management of thyroid cancer. Rambam Maimonides Med J 2014; 5 (02) e0008
- Wong KT, Ahuja AT. Ultrasound of thyroid cancer. Cancer Imaging 2005; 5 (01) 157-166
- Chng CL, Tan HC, Too CW. et al. Diagnostic performance of ATA, BTA and TIRADS sonographic patterns in the prediction of malignancy in histologically proven thyroid nodules. Singapore Med J 2018; 59 (11) 578-583
- Gharib H, Papini E, Garber JR. et al; American association of clinical endocrinologists, American college of endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update appendix. Endocrine practice 2016; 22: 1-60
- Lee YJ, Kim DW, Shin GW. et al. Comparison of ultrasonography features and K-TIRADS for isthmic and lobar papillary thyroid carcinomas: a single-center study. Front Endocrinol (Lausanne) 2020; 11: 328
- Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 2017; 6 (05) 225-237
- Tessler FN, Middleton WD, Grant EG. et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol 2017; 14 (05) 587-595
- Grani G, Lamartina L, Ascoli V. et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab 2019; 104 (01) 95-102
- Tessler FN, Middleton WD, Grant EG. et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Amer Coll Radiol 2017; 14 (05) 587-595
- Hoang JK, Branstetter IV BF, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging 2013; 13 (01) 128-139
- Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg 2000; 24 (08) 942-951
- King AD. Imaging for staging and management of thyroid cancer. Cancer Imaging 2008; 8 (01) 57-69
- Ahuja AT, Chow L, Chick W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol 1995; 50 (04) 229-231
- Yeh MW, Bauer AJ, Bernet VA. et al; American Thyroid Association Surgical Affairs Committee Writing Task Force. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 2015; 25 (01) 3-14
- Kim MJ, Kim EK, Park SI. et al. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics 2008; 28 (07) 1869-1886 , discussion 1887
- Bhatki AM, Brewer B, Robinson-Smith T, Nikiforov Y, Steward DL. Adequacy of surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy. Otolaryngol Head Neck Surg 2008; 139 (01) 27-31
- Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab 2006; 91 (04) 1364-1369
- Snozek CL, Chambers EP, Reading CC. et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab 2007; 92 (11) 4278-4281
- Boi F, Maurelli I, Pinna G. et al. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 2007; 92 (06) 2115-2118
- Tran Cao HS, Johnston LE, Chang DC, Bouvet M. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 2012; 152 (02) 145-151
- Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American Joint Committee on cancer TNM Staging system for medullary thyroid cancer. JAMA Surg 2017; 152 (09) 869-876
- Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2018; 68 (01) 55-63
- Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg 2007; 245 (03) 366-378
- Adam MA, Pura J, Goffredo P. et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 2015; 33 (21) 2370-2375
- Zaheer S, Tan A, Ang ES. et al. Post-thyroidectomy neck ultrasonography in patients with thyroid cancer and a review of the literature. Singapore Med J 2014; 55 (04) 177-182 , quiz 183
- Schlumberger M, Berg G, Cohen O. et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol 2004; 150 (02) 105-112
- Pacini F, Molinaro E, Castagna MG. et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003; 88 (08) 3668-3673
- Wang LY, Ganly I. Post-treatment surveillance of thyroid cancer. Eur J Surg Oncol 2018; 44 (03) 357-366
- Ito Y, Miyauchi A. Active surveillance of low-risk papillary thyroid microcarcinomas. Gland Surg 2020; 9 (05) 1663-1673
- Bunch PM, Kelly HR. Preoperative imaging techniques in primary hyperparathyroidism: a review. JAMA Otolaryngol Head Neck Surg 2018; 144 (10) 929-937
- Fraser WD. Hyperparathyroidism. Lancet 2009; 374 (9684): 145-158
- Ruda JM, Hollenbeak CS, Stack Jr BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005; 132 (03) 359-372
- Yeh MW, Ituarte PH, Zhou HC. et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 2013; 98 (03) 1122-1129
- Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet 2018; 391 (10116): 168-178
- Machado NN, Wilhelm SM. Diagnosis and evaluation of primary hyperparathyroidism. Surg Clin North Am 2019; 99 (04) 649-666
- Palmér M, Adami HO, Bergström R, Jakobsson S, Akerström G, Ljunghall S. Survival and renal function in untreated hypercalcaemia. Population-based cohort study with 14 years of follow-up. Lancet 1987; 1 (8524): 59-62
- Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 1987; 102 (01) 1-7
- Hedbäck G, Tisell LE, Bengtsson BÅ, Hedman I, Oden A. Premature death in patients operated on for primary hyperparathyroidism. World J Surg 1990; 14 (06) 829-835 , discussion 836
- Leifsson BG, Ahrén B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab 1996; 81 (06) 2149-2153
- Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism—an update. Eur J Clin Invest 1998; 28 (04) 271-276
- Yu N, Donnan PT, Flynn RW. et al; The Parathyroid Epidemiology and Audit Research Study (PEARS). Increased mortality and morbidity in mild primary hyperparathyroid patients. Clin Endocrinol (Oxf) 2010; 73 (01) 30-34
- Zanocco K, Angelos P, Sturgeon C. Cost-effectiveness analysis of parathyroidectomy for asymptomatic primary hyperparathyroidism. Surgery 2006; 140 (06) 874-881 , discussion 881–882
- Zanocco KA, Wu JX, Yeh MW. Parathyroidectomy for asymptomatic primary hyperparathyroidism: a revised cost-effectiveness analysis incorporating fracture risk reduction. Surgery 2017; 161 (01) 16-24
- Bunch PM, Randolph GW, Brooks JA, George V, Cannon J, Kelly HR. Parathyroid 4D CT: what the surgeon wants to know. Radiographics 2020; 40 (05) 1383-1394
- Rodgers SE, Hunter GJ, Hamberg LM. et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006; 140 (06) 932-940 , discussion 940–941
- Suh YJ, Choi JY, Kim SJ. et al. Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism. Otolaryngol Head Neck Surg 2015; 152 (03) 438-443
- Kelly HR, Hamberg LM, Hunter GJ. 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naive and re-exploration patients. AJNR Am J Neuroradiol 2014; 35 (01) 176-181
- Tian Y, Tanny ST, Einsiedel P. et al. Four-dimensional computed tomography: clinical impact for patients with primary hyperparathyroidism. Ann Surg Oncol 2018; 25 (01) 117-121
- Yeh R, Tay YD, Tabacco G. et al. Diagnostic performance of 4D CT and Sestamibi SPECT/CT in localizing parathyroid adenomas in primary hyperparathyroidism. Radiology 2019; 291 (02) 469-476
- Bahl M, Sepahdari AR, Sosa JA, Hoang JK. Parathyroid adenomas and hyperplasia on four-dimensional CT scans: three patterns of enhancement relative to the thyroid gland justify a three-phase protocol. Radiology 2015; 277 (02) 454-462
- Sho S, Yilma M, Yeh MW. et al. Prospective validation of two 4D-CT-based scoring systems for prediction of multigland disease in primary hyperparathyroidism. AJNR Am J Neuroradiol 2016; 37 (12) 2323-2327
- Raghavan P, Durst CR, Ornan DA. et al. Dynamic CT for parathyroid disease: are multiple phases necessary?. AJNR Am J Neuroradiol 2014; 35 (10) 1959-1964
- Griffith B, Chaudhary H, Mahmood G. et al. Accuracy of 2-phase parathyroid CT for the preoperative localization of parathyroid adenomas in primary hyperparathyroidism. AJNR Am J Neuroradiol 2015; 36 (12) 2373-2379
- Jason DS, Balentine CJ. Intraoperative decision making in parathyroid surgery. Surg Clin North Am 2019; 99 (04) 681-691
- Wilhelm SM, Wang TS, Ruan DT. et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016; 151 (10) 959-968
- Minisola S, Cipriani C, Diacinti D. et al. Imaging of the parathyroid glands in primary hyperparathyroidism. Eur J Endocrinol 2016; 174 (01) D1-D8
- Parangi S, Pandian TK, Thompson G. Minimally invasive single gland parathyroid exploration. Surgery of the thyroid and parathyroid glands. Amsterdam, the Netherlands: Elsevier; 2021: 529-536
- Stack Jr BC, Tolley NS, Bartel TB. et al. AHNS Series: do you know your guidelines? Optimizing outcomes in reoperative parathyroid surgery: definitive multidisciplinary joint consensus guidelines of the American Head and Neck Society and the British Association of Endocrine and Thyroid Surgeons. Head Neck 2018; 40 (08) 1617-1629
- Schneider AB, Lubin J, Ron E. et al. Salivary gland tumors after childhood radiation treatment for benign conditions of the head and neck: dose-response relationships. Radiat Res 1998; 149 (06) 625-630
- Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol 2010; 74 (02) 134-148
- Forrest J, Campbell P, Kreiger N, Sloan M. Salivary gland cancer: an exploratory analysis of dietary factors. Nutr Cancer 2008; 60 (04) 469-473
- Kessler AT, Bhatt AA. Review of the major and minor salivary glands, part 2: neoplasms and tumor-like lesions. J Clin Imaging Sci 2018; 8: 48
- Freling N, Crippa F, Maroldi R. Staging and follow-up of high-grade malignant salivary gland tumours: the role of traditional versus functional imaging approaches—a review. Oral Oncol 2016; 60: 157-166
- Ferlay J, Ervik M, Lam F. et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020
- Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging 2007; 7 (01) 52-62
- Wierzbicka M, Kopeć T, Szyfter W, Kereiakes T, Bem G. The presence of facial nerve weakness on diagnosis of a parotid gland malignant process. Eur Arch Otorhinolaryngol 2012; 269 (04) 1177-1182
- Mantravadi AV, Moore MG, Rassekh CH. AHNS series: do you know your guidelines? Diagnosis and management of salivary gland tumors. Head Neck 2019; 41 (02) 269-280
- Pfister DG, Spencer S, Adelstein D. et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020; 18 (07) 873-898
- Zheng N, Li R, Liu W, Shao S, Jiang S. The diagnostic value of combining conventional, diffusion-weighted imaging and dynamic contrast-enhanced MRI for salivary gland tumors. Br J Radiol 2018; 91 (1089): 20170707
- Ma G, Zhu LN, Su GY. et al. Histogram analysis of apparent diffusion coefficient maps for differentiating malignant from benign parotid gland tumors. Eur Arch Otorhinolaryngol 2018; 275 (08) 2151-2157
- Geiger JL, Ismaila N, Beadle B. et al. Management of salivary gland malignancy: ASCO guideline. J Clin Oncol 2021; 39 (17) 1909-1941
- Cermik TF, Mavi A, Acikgoz G, Houseni M, Dadparvar S, Alavi A. FDG PET in detecting primary and recurrent malignant salivary gland tumors. Clin Nucl Med 2007; 32 (04) 286-291
- WERING B. Neoplasms of the salivary glands. Atlas of head and neck pathology. 2008:582–91.
- Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol 2008; 66 (03) 419-436
- Terhaard CH, Lubsen H, Van der Tweel I. et al; Dutch Head and Neck Oncology Cooperative Group. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2004; 26 (08) 681-692 , discussion 692–693
- Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H. MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 2011; 32 (07) 1202-1207
- Habermann CR, Arndt C, Graessner J. et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible?. AJNR Am J Neuroradiol 2009; 30 (03) 591-596
- Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 2003; 226 (02) 345-354
- Kim JY, Lee SW, Kim JS. et al. Diagnostic value of neck node status using 18F-FDG PET for salivary duct carcinoma of the major salivary glands. J Nucl Med 2012; 53 (06) 881-886
- Digonnet A, Hamoir M, Andry G. et al. Follow-up strategies in head and neck cancer other than upper aerodigestive tract squamous cell carcinoma. Eur Arch Otorhinolaryngol 2013; 270 (07) 1981-1989
- Manikantan K, Khode S, Dwivedi RC. et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev 2009; 35 (08) 744-753
- Hermans R. Posttreatment imaging in head and neck cancer. Eur J Radiol 2008; 66 (03) 501-511
- Razfar A,
Heron DE, Branstetter IV BF, Seethala RR, Ferris
RL. Positron emission
tomography-computed tomography adds to the management of salivary gland malignancies. Laryngoscope 2010; 120
(04) 734-738
Address for correspondence
Abhishek Mahajan, MD, Fellowship in Cancer Imaging, MRes (KCL, London), FRCR (UK)Department of Radiodiagnosis, The Clatterbridge Cancer Centre NHS Foundation TrustPembroke Place Liverpool, Liverpool L7 8YAUnited KingdomEmail: drabhishek.mahajan@yahoo.inPublication History
Article published online:
04 May 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Imaging recommendations by NCCN guidelines.
References
- Tufano RP, Clayman G, Heller KS. et al; American Thyroid Association Surgical Affairs Committee Writing Task Force. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid 2015; 25 (01) 15-27
- Noone AM, Cronin KA, Altekruse SF. et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomarkers Prev 2017; 26 (04) 632-641
- John AM, Jacob PM, Oommen R, Nair S, Nair A, Rajaratnam S. Our experience with papillary thyroid microcancer. Indian J Endocrinol Metab 2014; 18 (03) 410-413
- Liu Y, Su L, Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol 2017; 2017: 5308635
- Crnčić TB, Tomaš MI, Girotto N, Ivanković SG. Risk factors for thyroid cancer: what do we know so far?. Acta Clin Croat 2020; 59 (Suppl. 01) 66-72
- Bonnefond S, Davies TF. Thyroid cancer-risks and causes. Oncol Hematol Rev 2014; 10 (02) 14451
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013; 13 (03) 184-199
- Patel KN, Yip L, Lubitz CC. et al. The American Association of Endocrine Surgeons Guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg 2020; 271 (03) e21-e93
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- O'Neill CJ, Oucharek J, Learoyd D, Sidhu SB. Standard and emerging therapies for metastatic differentiated thyroid cancer. Oncologist 2010; 15 (02) 146-156
- Lechner MG, Hershman JM. Thyroid Nodules and Cancer in the Elderly. In: Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. PMID: 25905203.
- Lee JY, Baek JH, Ha EJ. et al; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2020 imaging guidelines for thyroid nodules and differentiated thyroid cancer: Korean Society of Thyroid Radiology. Korean J Radiol 2021; 22 (05) 840-860
- Haugen BR, Alexander EK, Bible KC. et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26 (01) 1-133
- Gharib H, Papini E, Paschke R. et al; AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive Summary of recommendations. J Endocrinol Invest 2010; 33 (5, Suppl): 287-291
- Perros P, Boelaert K, Colley S. et al; British Thyroid Association. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014; 81 (Suppl. 01) 1-122
- Shin JH, Baek JH, Chung J. et al; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2016; 17 (03) 370-395
- Yi KH, Park YJ, Koong SS. et al. Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Endocrinol Metab (Seoul) 2010; 25 (04) 270-297
- www.nccn.org
- Wells Jr SAJ, Asa SL, Dralle H. et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25 (06) 567-610
- Bible KC, Kebebew E, Brierley J. et al. 2021 American thyroid association guidelines for management of patients with anaplastic thyroid cancer: American thyroid association anaplastic thyroid cancer guidelines task force. Thyroid 2021; 31 (03) 337-386
- Seib CD, Harari A, Conte FA, Duh QY, Clark OH, Gosnell JE. Utility of serum thyroglobulin measurements after prophylactic thyroidectomy in patients with hereditary medullary thyroid cancer. Surgery 2014; 156 (02) 394-398
- Cibas ES, Ali SZ. The 2017 Bethesda System for reporting thyroid cytopathology. Thyroid 2017; 27 (11) 1341-1346
- Birtwhistle R, Morissette K, Dickinson JA. et al; Canadian Task Force on Preventive Health Care. Recommendation on screening adults for asymptomatic thyroid dysfunction in primary care. CMAJ 2019; 191 (46) E1274-E1280
- Morrison SA, Suh H, Hodin RA. The surgical management of thyroid cancer. Rambam Maimonides Med J 2014; 5 (02) e0008
- Wong KT, Ahuja AT. Ultrasound of thyroid cancer. Cancer Imaging 2005; 5 (01) 157-166
- Chng CL, Tan HC, Too CW. et al. Diagnostic performance of ATA, BTA and TIRADS sonographic patterns in the prediction of malignancy in histologically proven thyroid nodules. Singapore Med J 2018; 59 (11) 578-583
- Gharib H, Papini E, Garber JR. et al; American association of clinical endocrinologists, American college of endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update appendix. Endocrine practice 2016; 22: 1-60
- Lee YJ, Kim DW, Shin GW. et al. Comparison of ultrasonography features and K-TIRADS for isthmic and lobar papillary thyroid carcinomas: a single-center study. Front Endocrinol (Lausanne) 2020; 11: 328
- Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 2017; 6 (05) 225-237
- Tessler FN, Middleton WD, Grant EG. et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol 2017; 14 (05) 587-595
- Grani G, Lamartina L, Ascoli V. et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab 2019; 104 (01) 95-102
- Tessler FN, Middleton WD, Grant EG. et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Amer Coll Radiol 2017; 14 (05) 587-595
- Hoang JK, Branstetter IV BF, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging 2013; 13 (01) 128-139
- Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg 2000; 24 (08) 942-951
- King AD. Imaging for staging and management of thyroid cancer. Cancer Imaging 2008; 8 (01) 57-69
- Ahuja AT, Chow L, Chick W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol 1995; 50 (04) 229-231
- Yeh MW, Bauer AJ, Bernet VA. et al; American Thyroid Association Surgical Affairs Committee Writing Task Force. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 2015; 25 (01) 3-14
- Kim MJ, Kim EK, Park SI. et al. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics 2008; 28 (07) 1869-1886 , discussion 1887
- Bhatki AM, Brewer B, Robinson-Smith T, Nikiforov Y, Steward DL. Adequacy of surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy. Otolaryngol Head Neck Surg 2008; 139 (01) 27-31
- Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab 2006; 91 (04) 1364-1369
- Snozek CL, Chambers EP, Reading CC. et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab 2007; 92 (11) 4278-4281
- Boi F, Maurelli I, Pinna G. et al. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 2007; 92 (06) 2115-2118
- Tran Cao HS, Johnston LE, Chang DC, Bouvet M. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 2012; 152 (02) 145-151
- Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American Joint Committee on cancer TNM Staging system for medullary thyroid cancer. JAMA Surg 2017; 152 (09) 869-876
- Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2018; 68 (01) 55-63
- Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg 2007; 245 (03) 366-378
- Adam MA, Pura J, Goffredo P. et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 2015; 33 (21) 2370-2375
- Zaheer S, Tan A, Ang ES. et al. Post-thyroidectomy neck ultrasonography in patients with thyroid cancer and a review of the literature. Singapore Med J 2014; 55 (04) 177-182 , quiz 183
- Schlumberger M, Berg G, Cohen O. et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol 2004; 150 (02) 105-112
- Pacini F, Molinaro E, Castagna MG. et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003; 88 (08) 3668-3673
- Wang LY, Ganly I. Post-treatment surveillance of thyroid cancer. Eur J Surg Oncol 2018; 44 (03) 357-366
- Ito Y, Miyauchi A. Active surveillance of low-risk papillary thyroid microcarcinomas. Gland Surg 2020; 9 (05) 1663-1673
- Bunch PM, Kelly HR. Preoperative imaging techniques in primary hyperparathyroidism: a review. JAMA Otolaryngol Head Neck Surg 2018; 144 (10) 929-937
- Fraser WD. Hyperparathyroidism. Lancet 2009; 374 (9684): 145-158
- Ruda JM, Hollenbeak CS, Stack Jr BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005; 132 (03) 359-372
- Yeh MW, Ituarte PH, Zhou HC. et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 2013; 98 (03) 1122-1129
- Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet 2018; 391 (10116): 168-178
- Machado NN, Wilhelm SM. Diagnosis and evaluation of primary hyperparathyroidism. Surg Clin North Am 2019; 99 (04) 649-666
- Palmér M, Adami HO, Bergström R, Jakobsson S, Akerström G, Ljunghall S. Survival and renal function in untreated hypercalcaemia. Population-based cohort study with 14 years of follow-up. Lancet 1987; 1 (8524): 59-62
- Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 1987; 102 (01) 1-7
- Hedbäck G, Tisell LE, Bengtsson BÅ, Hedman I, Oden A. Premature death in patients operated on for primary hyperparathyroidism. World J Surg 1990; 14 (06) 829-835 , discussion 836
- Leifsson BG, Ahrén B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab 1996; 81 (06) 2149-2153
- Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism—an update. Eur J Clin Invest 1998; 28 (04) 271-276
- Yu N, Donnan PT, Flynn RW. et al; The Parathyroid Epidemiology and Audit Research Study (PEARS). Increased mortality and morbidity in mild primary hyperparathyroid patients. Clin Endocrinol (Oxf) 2010; 73 (01) 30-34
- Zanocco K, Angelos P, Sturgeon C. Cost-effectiveness analysis of parathyroidectomy for asymptomatic primary hyperparathyroidism. Surgery 2006; 140 (06) 874-881 , discussion 881–882
- Zanocco KA, Wu JX, Yeh MW. Parathyroidectomy for asymptomatic primary hyperparathyroidism: a revised cost-effectiveness analysis incorporating fracture risk reduction. Surgery 2017; 161 (01) 16-24
- Bunch PM, Randolph GW, Brooks JA, George V, Cannon J, Kelly HR. Parathyroid 4D CT: what the surgeon wants to know. Radiographics 2020; 40 (05) 1383-1394
- Rodgers SE, Hunter GJ, Hamberg LM. et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006; 140 (06) 932-940 , discussion 940–941
- Suh YJ, Choi JY, Kim SJ. et al. Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism. Otolaryngol Head Neck Surg 2015; 152 (03) 438-443
- Kelly HR, Hamberg LM, Hunter GJ. 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naive and re-exploration patients. AJNR Am J Neuroradiol 2014; 35 (01) 176-181
- Tian Y, Tanny ST, Einsiedel P. et al. Four-dimensional computed tomography: clinical impact for patients with primary hyperparathyroidism. Ann Surg Oncol 2018; 25 (01) 117-121
- Yeh R, Tay YD, Tabacco G. et al. Diagnostic performance of 4D CT and Sestamibi SPECT/CT in localizing parathyroid adenomas in primary hyperparathyroidism. Radiology 2019; 291 (02) 469-476
- Bahl M, Sepahdari AR, Sosa JA, Hoang JK. Parathyroid adenomas and hyperplasia on four-dimensional CT scans: three patterns of enhancement relative to the thyroid gland justify a three-phase protocol. Radiology 2015; 277 (02) 454-462
- Sho S, Yilma M, Yeh MW. et al. Prospective validation of two 4D-CT-based scoring systems for prediction of multigland disease in primary hyperparathyroidism. AJNR Am J Neuroradiol 2016; 37 (12) 2323-2327
- Raghavan P, Durst CR, Ornan DA. et al. Dynamic CT for parathyroid disease: are multiple phases necessary?. AJNR Am J Neuroradiol 2014; 35 (10) 1959-1964
- Griffith B, Chaudhary H, Mahmood G. et al. Accuracy of 2-phase parathyroid CT for the preoperative localization of parathyroid adenomas in primary hyperparathyroidism. AJNR Am J Neuroradiol 2015; 36 (12) 2373-2379
- Jason DS, Balentine CJ. Intraoperative decision making in parathyroid surgery. Surg Clin North Am 2019; 99 (04) 681-691
- Wilhelm SM, Wang TS, Ruan DT. et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016; 151 (10) 959-968
- Minisola S, Cipriani C, Diacinti D. et al. Imaging of the parathyroid glands in primary hyperparathyroidism. Eur J Endocrinol 2016; 174 (01) D1-D8
- Parangi S, Pandian TK, Thompson G. Minimally invasive single gland parathyroid exploration. Surgery of the thyroid and parathyroid glands. Amsterdam, the Netherlands: Elsevier; 2021: 529-536
- Stack Jr BC, Tolley NS, Bartel TB. et al. AHNS Series: do you know your guidelines? Optimizing outcomes in reoperative parathyroid surgery: definitive multidisciplinary joint consensus guidelines of the American Head and Neck Society and the British Association of Endocrine and Thyroid Surgeons. Head Neck 2018; 40 (08) 1617-1629
- Schneider AB, Lubin J, Ron E. et al. Salivary gland tumors after childhood radiation treatment for benign conditions of the head and neck: dose-response relationships. Radiat Res 1998; 149 (06) 625-630
- Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol 2010; 74 (02) 134-148
- Forrest J, Campbell P, Kreiger N, Sloan M. Salivary gland cancer: an exploratory analysis of dietary factors. Nutr Cancer 2008; 60 (04) 469-473
- Kessler AT, Bhatt AA. Review of the major and minor salivary glands, part 2: neoplasms and tumor-like lesions. J Clin Imaging Sci 2018; 8: 48
- Freling N, Crippa F, Maroldi R. Staging and follow-up of high-grade malignant salivary gland tumours: the role of traditional versus functional imaging approaches—a review. Oral Oncol 2016; 60: 157-166
- Ferlay J, Ervik M, Lam F. et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020
- Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging 2007; 7 (01) 52-62
- Wierzbicka M, Kopeć T, Szyfter W, Kereiakes T, Bem G. The presence of facial nerve weakness on diagnosis of a parotid gland malignant process. Eur Arch Otorhinolaryngol 2012; 269 (04) 1177-1182
- Mantravadi AV, Moore MG, Rassekh CH. AHNS series: do you know your guidelines? Diagnosis and management of salivary gland tumors. Head Neck 2019; 41 (02) 269-280
- Pfister DG, Spencer S, Adelstein D. et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020; 18 (07) 873-898
- Zheng N, Li R, Liu W, Shao S, Jiang S. The diagnostic value of combining conventional, diffusion-weighted imaging and dynamic contrast-enhanced MRI for salivary gland tumors. Br J Radiol 2018; 91 (1089): 20170707
- Ma G, Zhu LN, Su GY. et al. Histogram analysis of apparent diffusion coefficient maps for differentiating malignant from benign parotid gland tumors. Eur Arch Otorhinolaryngol 2018; 275 (08) 2151-2157
- Geiger JL, Ismaila N, Beadle B. et al. Management of salivary gland malignancy: ASCO guideline. J Clin Oncol 2021; 39 (17) 1909-1941
- Cermik TF, Mavi A, Acikgoz G, Houseni M, Dadparvar S, Alavi A. FDG PET in detecting primary and recurrent malignant salivary gland tumors. Clin Nucl Med 2007; 32 (04) 286-291
- WERING B. Neoplasms of the salivary glands. Atlas of head and neck pathology. 2008:582–91.
- Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol 2008; 66 (03) 419-436
- Terhaard CH, Lubsen H, Van der Tweel I. et al; Dutch Head and Neck Oncology Cooperative Group. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2004; 26 (08) 681-692 , discussion 692–693
- Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H. MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 2011; 32 (07) 1202-1207
- Habermann CR, Arndt C, Graessner J. et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible?. AJNR Am J Neuroradiol 2009; 30 (03) 591-596
- Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 2003; 226 (02) 345-354
- Kim JY, Lee SW, Kim JS. et al. Diagnostic value of neck node status using 18F-FDG PET for salivary duct carcinoma of the major salivary glands. J Nucl Med 2012; 53 (06) 881-886
- Digonnet A, Hamoir M, Andry G. et al. Follow-up strategies in head and neck cancer other than upper aerodigestive tract squamous cell carcinoma. Eur Arch Otorhinolaryngol 2013; 270 (07) 1981-1989
- Manikantan K, Khode S, Dwivedi RC. et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev 2009; 35 (08) 744-753
- Hermans R. Posttreatment imaging in head and neck cancer. Eur J Radiol 2008; 66 (03) 501-511
- Razfar A, Heron DE, Branstetter IV BF, Seethala RR, Ferris RL. Positron emission tomography-computed tomography adds to the management of salivary gland malignancies. Laryngoscope 2010; 120 (04) 734-738


 PDF
PDF  Views
Views  Share
Share

