Imaging Recommendations for Diagnosis, Staging and Management of Larynx and Hypopharynx Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 054-065
DOI: DOI: 10.1055/s-0042-1759504
Abstract
We discussed the imaging recommendations for diagnosis, staging, and management of larynx and hypopharynx cancer. Carcinoma of the larynx is a common cancer, with males being affected more. Hypopharyngeal carcinoma is less common than laryngeal malignancies. Squamous cell carcinoma is the most common histological type. Nonsquamous cell malignant lesions are rare and mostly submucosal lesions. Clinical examination and endoscopy play an integral role in its detection and staging. Imaging also plays a major role in its staging, including local disease extent, nodal and distant metastatic status, as well as to assess response to therapy. Follow-up of treated cases and differentiation of recurrence from post treatment changes can be done on imaging. Early stage disease is treated with single modalities such as radiotherapy or surgery. Advanced disease is treated with multimodality of either chemoradiotherapy or surgery followed by adjuvant radiotherapy with or without concurrent chemotherapy.
Note
The article is not under consideration for publication elsewhere. Each author participated sufficiently for the work to be submitted. Publication is approved by all authors.
Publication History
Article published online:
24 January 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We discussed the imaging recommendations for diagnosis, staging, and management of larynx and hypopharynx cancer. Carcinoma of the larynx is a common cancer, with males being affected more. Hypopharyngeal carcinoma is less common than laryngeal malignancies. Squamous cell carcinoma is the most common histological type. Nonsquamous cell malignant lesions are rare and mostly submucosal lesions. Clinical examination and endoscopy play an integral role in its detection and staging. Imaging also plays a major role in its staging, including local disease extent, nodal and distant metastatic status, as well as to assess response to therapy. Follow-up of treated cases and differentiation of recurrence from post treatment changes can be done on imaging. Early stage disease is treated with single modalities such as radiotherapy or surgery. Advanced disease is treated with multimodality of either chemoradiotherapy or surgery followed by adjuvant radiotherapy with or without concurrent chemotherapy.
Introduction
Carcinoma of the larynx is the second most common head and neck cancer after oral cavity. It is commonly diagnosed in patients above 50 years of age. The incidence of this cancer is more in males.[1] Hypopharyngeal carcinoma is relatively less common than laryngeal malignancies.[2] Histologically, squamous cell carcinoma is the most common type. These cancers are commonly associated with lungs and aerodigestive malignancies due to common risk factors.[1]
Endoscopy can detect mucosal lesions; however, cross-sectional imaging (computed tomography and/or magnetic resonance imaging) improves the accuracy of loco-regional staging of the disease.[1] [2] [3] Submucosal extension of the disease including involvement of the spaces and cartilages cannot be assessed on endoscopy and therefore supplementation of the clinical examination with imaging is essential for appropriate treatment planning. Cross-sectional imaging also gives information about nodal status and distant metastases and helps in restaging and follow-up of treated patients. Distorted anatomy and post treatment fibrosis may make the clinical examination challenging. Functional imaging techniques such as PET CT play a crucial role in response assessment after organ preservation protocols.
Risk Factors and Etiopathogenesis
Smoking, tobacco, and alcohol are the major risk factors[2] [4]; others being indoor air pollution and meat rich, low-fiber diet.[5] [6]
These carcinomas are usually mucosal in origin and then infiltrate into the submucosal tissues. The anatomical barriers produced by the laryngeal compartments and intercartilaginous membranes restrict their initial spread by forming a rigid barrier, due to which they tend to spread along pathways of least resistance into the soft tissue. However, in the later stages, invasion of the bone and/or cartilage is seen.[2] A careful evaluation of the lungs must be done for concurrent primary bronchogenic malignancies.
Lymphatic spread to neck nodes is very common in supraglottic and hypopharyngeal cancers. Glottic carcinomas do not have nodal spread commonly,[2] being 0 to 10%-in early cancers and 10 to 35%-in advanced cases.[7] Distant metastasis is less common, ∼6.5 to 8.5%.[8] [9] [10] Lungs are the most common site of distant metastasis, followed by bones and liver.[2]
Epidemiology, Clinical Presentation in India and Global
Laryngeal cancer forms 2%-of all cancers with more than 1,59,000 new cases and 90,000 cancer deaths worldwide.[11] In India, laryngeal cancer contributes to ∼3 to 6%-of all cancers in men, varying in different regions of the country.[5] The 5-year survival for laryngeal cancer in India is ∼28%.[5]
The incidence of hypopharyngeal cancers is relatively higher in India (∼11%-against 1%-worldwide).[11] Dietary variation has been proposed as a likely cause of this difference.
Common presenting symptoms are hoarseness, difficulty in breathing, dysphagia, or odynophagia, foreign body sensation, ear ache, and advanced disease may lead to stridor or aspiration.[11]
Imaging Referral Guidelines
As per the NCCN (National Comprehensive Cancer Network) guidelines, version V.1.2021, the following investigations are to be done for patients with larynx or hypopharynx cancer[12]:
Examination under anesthesia with endoscopy.
CECT and/or MRI for primary and neck (thin angled cuts through larynx for laryngeal carcinoma).
Chest CT (with/without contrast) for advanced nodal disease to screen for distant metastasis and screen for lung cancer in smokers.
FDG PET-CT for stage III-IV disease.
Clinical and examination findings are suggestive of the diagnosis of laryngeal carcinoma. Direct laryngoscopy with a confirmatory biopsy establishes the diagnosis. Direct laryngoscopy helps to assess the mucosal extent of disease as well as certain areas that may not be amenable to examination in routine OPD examination such as ventricles, subglottis, pyriform sinus, and post cricoid area. Imaging complements endoscopy and helps in accurate assessment and staging of disease. Contrast-enhanced CT scan is the modality of choice for initial evaluation due to its wider availability, and cheaper and faster acquisition. CT is less prone to swallowing artifacts and provides better spatial resolution compared with MRI.[1] It may be complemented with MRI in cases with dilemma, especially related to cartilage involvement.
Contrast-enhanced MRI is better to assess early cartilage involvement, pre epiglottic space, and tongue base involvement. MRI offers advantages of higher contrast resolution, differentiates tumor from peri-tumoral inflammatory response, thus helping in accurate disease assessment.[1] MRI has a higher sensitivity (89–96%) and a higher accuracy (84–76%) in detecting cartilage erosion,[1] [13] but has lower specificity (74–84%) as compared with CT.[1] It has disadvantage of images being degraded by motion artifact and larger acquisition times.
FDG-PET CT is done in cases of advanced loco-regional disease to assess distant metastases. PET CT is also more helpful than CT and MRI to differentiate recurrence from treatment-related changes.[14]
All patients diagnosed with hypopharyngeal carcinoma should also be imaged because submucosal spread is common, and hence volume of disease can be underdiagnosed on endoscopy. Contrast-enhanced CT or MRI is used for detecting the extent of disease and its size. FDG PET CT is especially useful in differentiating recurrence from post treatment changes such as in laryngeal cancers.[10]
Clinical/Diagnostic Work-up Excluding Imaging
Cancer of the larynx and hypopharynx differ in many clinical aspects. Laryngeal cancer is more common than hypopharyngeal cancer. Because hypopharyngeal cancer can be asymptomatic for a long time, these cases more frequently present with advanced cancers compared with laryngeal carcinoma. Nodal and systemic metastatic involvement is more common in hypopharyngeal than laryngeal carcinoma and neck mass may even be the first presentation in hypopharyngeal cancer. Relapse rates are also higher with carcinoma of the hypopharynx.[11] Despite these differences, the clinical/diagnostic work up is similar.
The clinical examination begins with a detailed evaluation of the primary lesion with an indirect laryngoscopy, which should be supplemented with the findings of direct laryngoscopy performed under general anesthesia. Indirect laryngoscopy is an office procedure that uses video camera or mirrors to indirectly visualize the lesion and assess cord/larynx mobility. Direct laryngoscopy directly visualizes the larynx and hypopharynx using rigid laryngoscopes.[15] Certain areas such asventricle, pyriform sinus apex, post cricoid area, and subglottis, which are suboptimally visible on indirect laryngoscopy are better visualized with direct laryngoscopy. Tissue biopsy is performed to establish the diagnosis. Neck should be meticulously examined for lymph nodes.[11]
Squamous cell carcinoma is the commonest epithelial tumor. Nonsquamous cell malignant lesions are rare and mostly submucosal lesions. These include chondrosarcoma, lymphoma, myelomas, and metastases. The clinical and endoscopic diagnosis of submucosal lesions is more difficult, and the initial biopsy if not taken from the submucosal lesion may be inconclusive or negative.[2] Imaging plays a strong role in detecting these lesions and can guide accurate biopsy in such cases.
Imaging Guidelines
a) Screening: There is no recommended screening test for laryngeal and hypopharyngeal cancers as of today.
b) Diagnosis: The CT and MRI protocol has been explained in [table1].[1] [2] [3] [16]
|
CT protocol |
MRI protocol |
|---|---|
|
Multidectector CT (MDCT, 16 slice or above) |
Multiplanar non-contrast T1-weighted, T2-weighted, T2-weighted fat-saturation images with postcontrast T1 fat-suppressed images |
|
Iodinated contrast agent (35–40 g iodine) injected at rate of 1–1.5 mL/s; with subsequent saline injection at the same rate |
Section thickness of 4 mm with an interslice gap of 0 to 1 mm |
|
Scan should be started after the entire contrast volume injected, ideally in the venous phase with a 60–90 s delay |
A dedicated neck coil |
|
In supine position with the patient breathing quietly; with patient instructed not to cough or swallow |
Instructions of not coughing and swallowing during the scan |
|
Axial images obtained from the skull base to the aortic arch; reconstruction parallel to the hyoid bone, to get images parallel to the true vocal cords. Additional maneuvers such as modified Valsalva or phonation for better visualization of the hypopharynx or laryngeal ventricle respectively. |
From the skull base to the thoracic inlet, with scan orientation parallel to the true vocal cords. |
|
Sagittal and coronal plane reformats important |
The pre-epiglottic space is better seen in the sagittal plane, while the paraglottic space and the ventricle are better assessed in the coronal plane. |
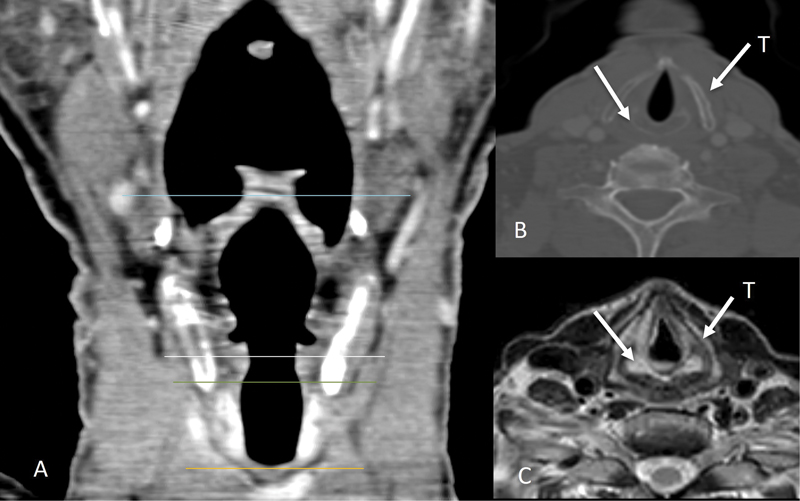
| Figure 1:Normal laryngeal anatomy. Coronal CT image (A) showing normal anatomy of the larynx and its subdivisions (supraglottis between blue and white lines; glottis between white and green lines; subglottis between the green and orange lines). Normal appearance of the thyroid cartilage (straight arrow labeled T) and cricoid cartilage (straight arrow) shown on image (B) and on axial T2W MRI image (C).
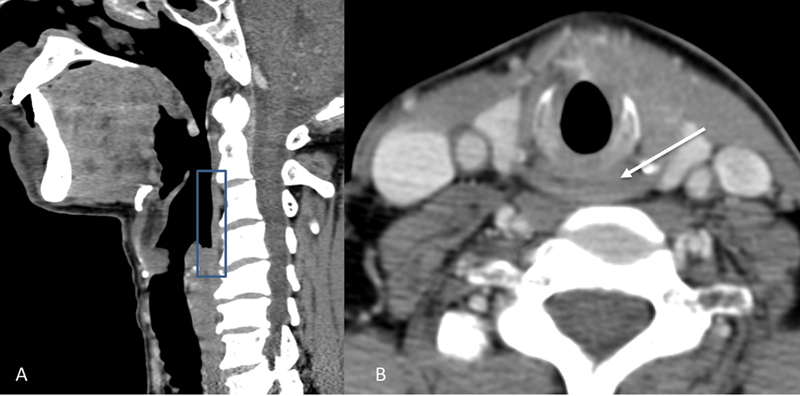
| Figure 2:Normal anatomy of the hypopharynx. Figure showing normal appearance of the hypopharynx (box) on sagittal CT image (A) and of the post cricoid region (arrow) on the axial CT image (B).
PET CT using fluorine-18-labeled 2-fluoro-2-deoxy-D-glucose (FDG) can be used in initial staging, especially in cases with advanced locoregional disease.[2] [3] [16] It can help locate distant metastases or synchronous primary malignancy. It has also proved superior to CT/MRI in initial staging of lymph node metastases.
Ultrasonography can detect the primary laryngeal tumor; however, artifacts caused by thyroid cartilage calcification and by air within the laryngeal cavities are unfavorable factors.[19] Ultrasound is an excellent modality to assess neck nodes in both laryngeal and hypopharyngeal carcinomas. It can also be used to guide fine needle aspiration cytology/biopsy from indeterminate neck nodes, nodal metastases being the most accurate prognostic factor for SCC.[2] [16] The appearance of a metastatic node on ultrasound is shown in [Fig. 3]. It can also be used for surveillance post treatment;[14] however, it is operator dependent.
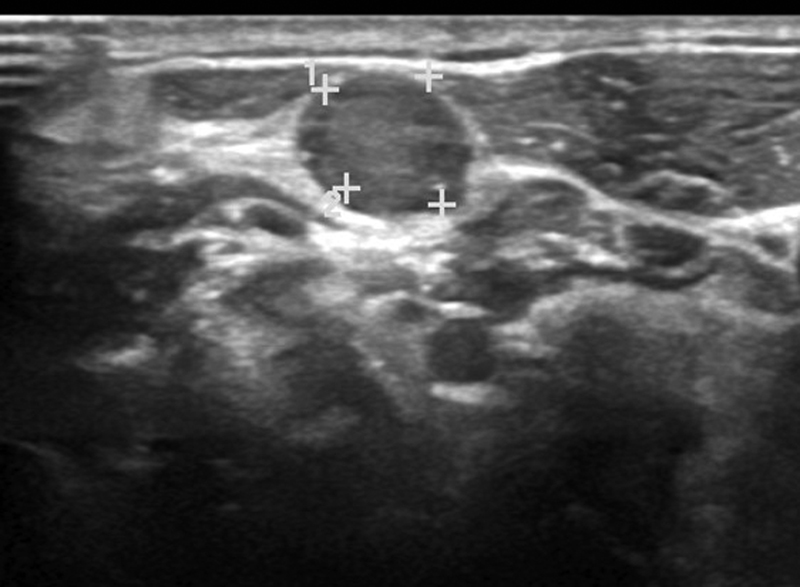
| Figure 3:Ultrasound image showing a round neck node with loss of fatty hilum, suggestive of an involved node.
The primary tumor can be detected as a soft tissue thickening or mass-forming disease showing abnormal contrast enhancement with or without infiltration of fatty tissue.[2] Associated inflammatory and edematous changes may overestimate the tumor extent. This is a common pitfall of CT imaging.
Carcinoma of the larynx arises in the supraglottic region (30%), glottis (65%), or subglottic region (5%).[1] [3]
Supraglottic laryngeal cancers tend to be detected in advanced stages because symptoms occur late. Supraglottic larynx has a rich lymphatic network; hence, nodal metastasis is a common finding in these patients. Supraglottic tumors may arise from the anterior components such as epiglottis, or the postero-lateral components such as aryepiglottic fold and false cords. The epiglottic lesions are usually seen along the midline anteriorly, that primarily invade into the pre-epiglottic space (PES) and laterally into the paraglottic space. The lesions arising from the stem of epiglottis often invade the low PES and then reach the glottis and subglottis via the anterior commissure. PES invasion is seen as replacement of the normal fat by abnormal enhancing soft tissue.[1] [2] [3] [Figs. 4] and [5] show examples of the imaging appearance of supraglottic cancer.

| Figure 4:Supraglottic laryngeal cancer. Axial and coronal CT images (A, C) showing a heterogeneously enhancing lesion (straight arrows) involving the left false vocal cord. The true vocal cords are normal in appearance and not involved as shown by the other axial image (B).
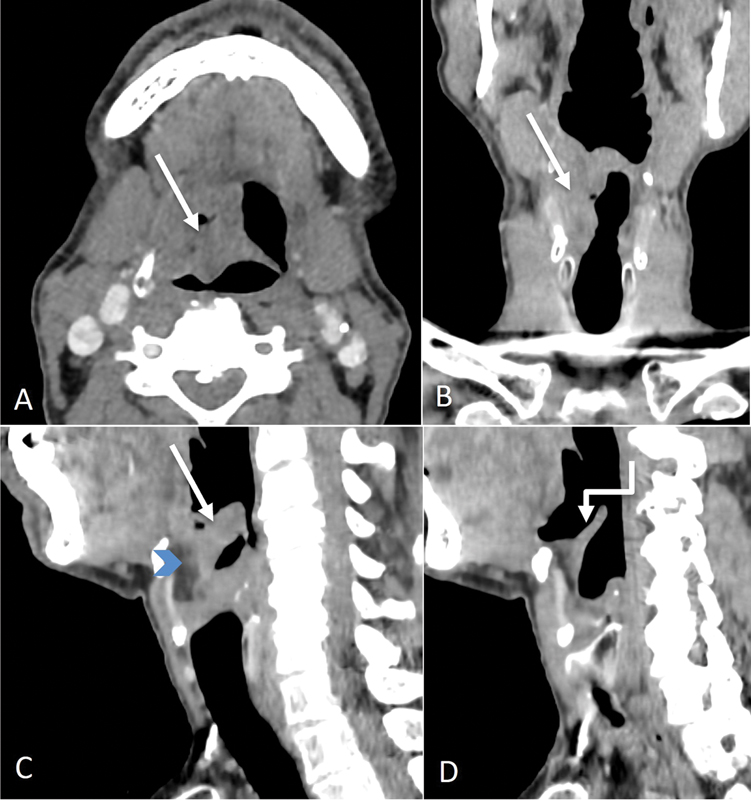
| Figure 5:Supraglottic laryngeal cancer. Axial, coronal, and sagittal CT images (A–C) showing the proliferative irregular mass (straight arrows) involving the right vallecula and supra and infrahyoid epiglottis on the right with involvement of the median glosso-epiglottic fold. There is involvement of the pre-epiglottic space also (blue arrowhead). Sagittal left paramedian CT image (D) showing normal uninvolved epiglottis on the left (shouldered arrow).
The commonest site of glottic cancer is the anterior aspect of vocal cord ([Fig. 6]). Involvement of the anterior commissure is common, and these lesions tend to cross to the contralateral vocal cord through the midline. Anterior commissural disease is seen on CT or MRI as soft tissue thickening of more than 1 to 2 mm. Lesions involving the anterior commissure may directly spread to the anterior subglottis, lower pre-epiglottic space. Tumors in the anterior commissure have a propensity to spread to the thyroid cartilage in the midline and hence advance to T3 stage quickly. When the tumor arises from the posterior vocal cord, posterior extension to arytenoid cartilage, and posterior commissure can occur. Further spread to the post cricoid hypopharynx and esophagus may occur. Laterally, the disease can involve the vocal ligament and muscle, followed by paraglottic space and thyroid cartilage. The tumor is diverted superiorly or inferiorly by the thyroid cartilage, into the paraglottic space or the subglottis respectively. Usually, glottic cancers metastasize to the neck lymph nodes when they have transglottic extension either into supraglottis or extension into subglottis.[1] [2] [3]
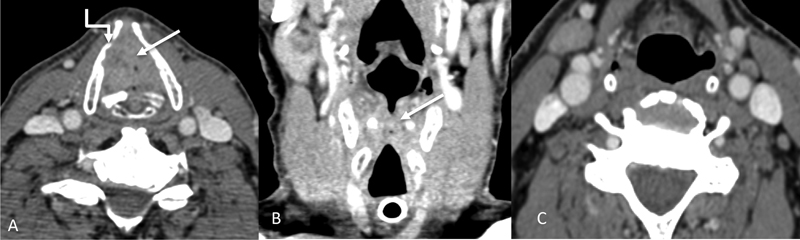
| Figure 6:Glottic laryngeal cancer. Axial and coronal CT images (A and B) showing a proliferative lesion (straight arrows) involving the true vocal cords bilaterally, with almost complete obliteration of the airway, necessitating a tracheostomy. The inner cortex of the right thyroid cartilage lamina is also involved (shouldered arrow) by the mass. No suspicious neck nodes were seen (C), which is usually the case in glottis cancers.
Subglottic cancers, although less common, are clinically silent and present late with a poor prognosis. Lymph node metastases are commonly seen and also affect the superior mediastinal nodes. Hence, the CT should be extended to include the pre and para-tracheal region of mediastinum in such patients. Subglottic cancer is detected by the presence of any mucosal thickening between the airway and the cricoid cartilage. Invasion of the cricoid cartilage, trachea, and the cervical esophagus with extra-laryngeal spread are common in these patients at presentation.[1] [3] [Figure 7] shows the imaging features of subglottic cancer. Apart from squamous cell carcinoma, adenoid cystic carcinoma is also common at the subglottic level.[2]
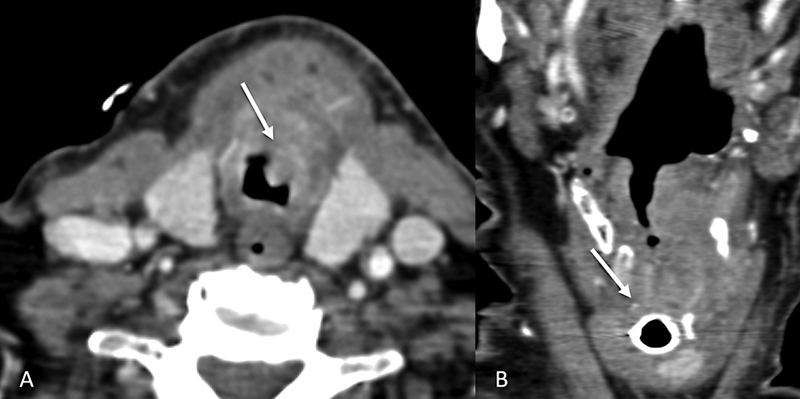
| Figure 7:Subglottic involvement. Axial and coronal CT images (A and B) showing subglottic involvement (straight arrows) of the laryngeal mass.
Transglottic cancer is when the disease involves both the glottis and supraglottis, irrespective of subglottic involvement.[1] [3] The CT appearance of transglottic cancer has been shown in [Figs. 8] and [9], and its appearance on MRI in [Fig. 10].
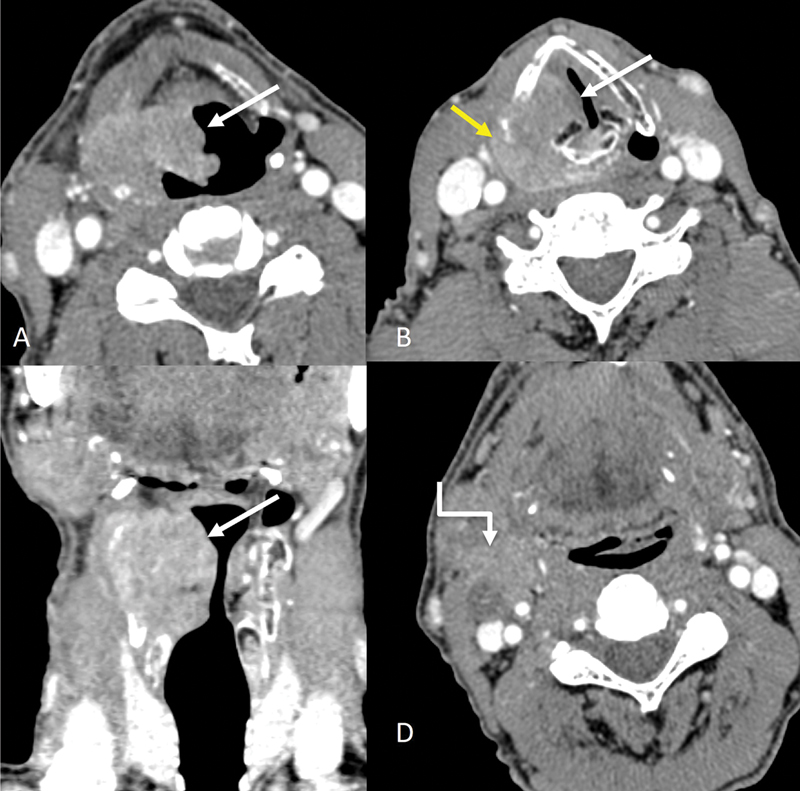
| Figure 8:Transglottic cancer involving supraglottis and glottis. Axial and coronal CT images (A–C) showing an enhancing exophytic mass (straight arrows) involving the right aryepiglottic fold and right vocal cord, with involvement of the right paraglottic space. Extralaryngeal spread of disease is seen (yellow arrow in B). An enlarged metastatic necrotic right level II node (shouldered arrow) is also seen (D).

| Figure 9:Transglottic laryngeal cancer. Axial CT images (A–C) showing the primary enhancing mass (straight arrows) involving the true vocal cords bilaterally and left false cord. There is gross involvement of the thyroid cartilage (more on the left side) with subtle extralaryngeal spread anteriorly (shouldered arrows). Right arytenoid cartilage appears sclerosed. The left para glottic space is also involved. In addition, lung nodules and liver lesions were also identified (D–F).
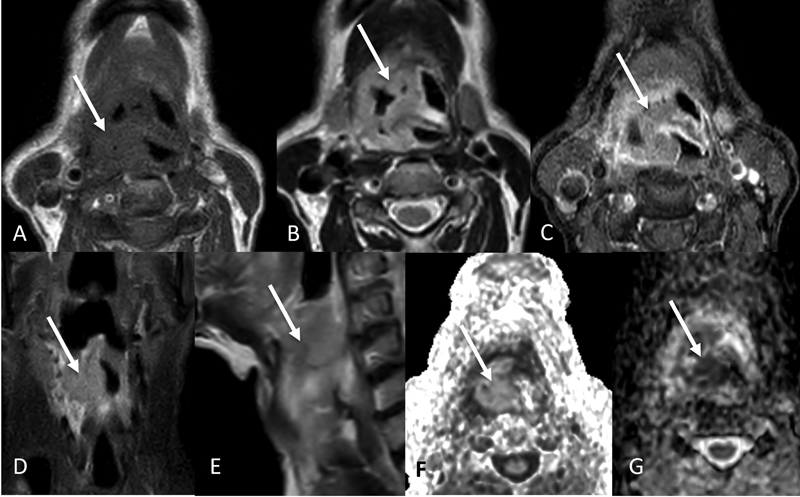
| Figure 10:Transglottic cancer. Axial T1W (A), T2W (B), post contrast T1W (C), coronal STIR (D), sagittal T2W (E) images showing the T1 isointense, T2 intermediate intensity, STIR hyperintense heterogeneously enhancing mass (arrows) involving the supraglottis and glottis. The mass shows restricted diffusion as seen on axial DWI and ADC images (F and G).
Gross cartilage invasion is easily detected with CT. Erosion (minor areas of osteolysis) or lysis (major areas of osteolysis) of the cartilages are pointers toward cartilage involvement, while extra-laryngeal spread is highly specific. Cartilage invasion on CT is demonstrated in [Fig. 11]. Because ossification pattern of the laryngeal cartilages is highly variable, CT can fail to detect early cartilage invasion. MRI is more sensitive, but not as specific, to detect cartilage abnormalities. Areas of cartilage involvement will be seen as increase in signal intensity on T2-weighted images and contrast-enhanced T1-weighted MRI images that matches the signal intensity of the tumor.[1] [2] [3] [16]
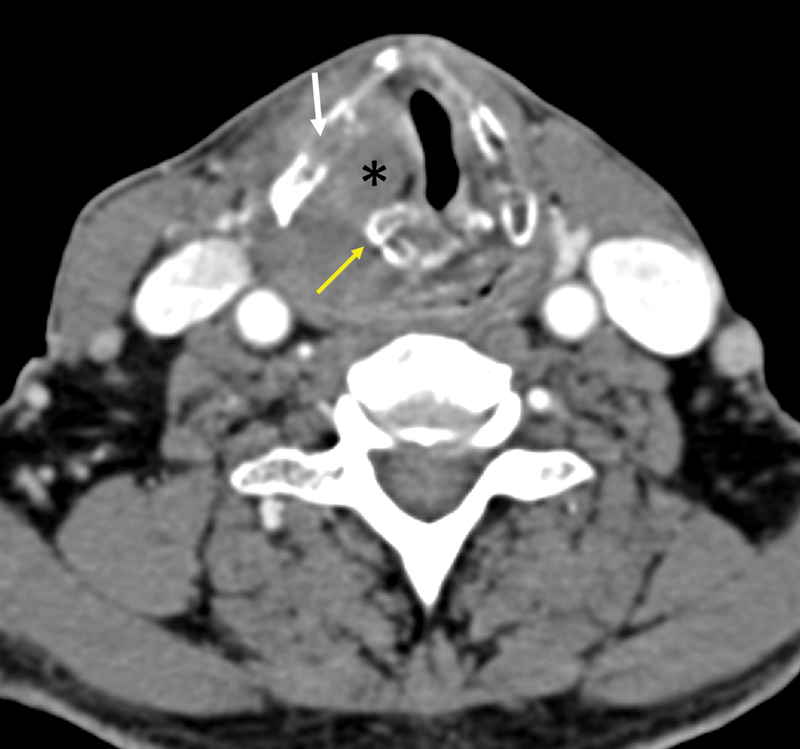
| Figure 11:Cartilage involvement-axial CT shows an enhancing mass (*) involving the right true cord, invading the thyroid cartilage on the right side (white arrow) and encasing the right arytenoid cartilage (yellow arrow).
Carcinoma of the hypopharynx may arise from the pyriform sinus (65%), postcricoid area (20%), and posterior pharyngeal wall (15%).[3] Imaging features of carcinoma hypopharynx have been shown in [Fig. 12].
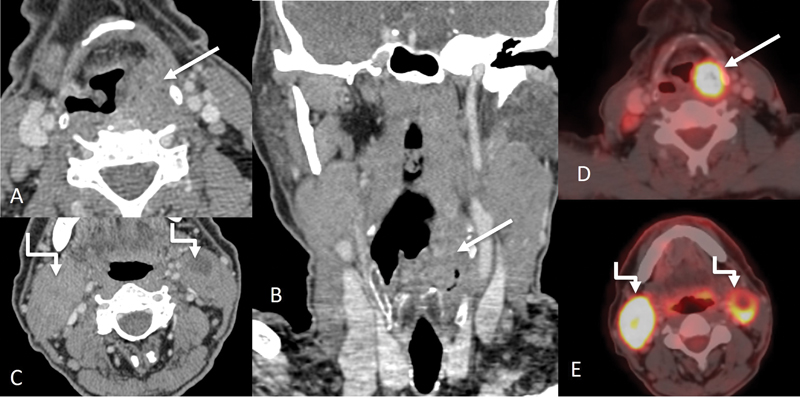
| Figure 12:Hypopharyngeal carcinoma involving pyriform sinus. Axial and coronal CT images (A, B) showing a proliferative mass (straight arrows) involving the left pyriform sinus. Enlarged metastatic bilateral level II nodes (shouldered arrow) are also seen on the axial CT image (C). Corresponding PET CT images (D and E) show significant uptake in the primary mass and metastatic neck nodes.
Tumors arising from the medial wall of the pyriform sinus tend to spread anteriorly into larynx through the paraglottic space. Tumors epicentered in the lateral wall of the pyriform sinus commonly infiltrate the soft tissues of the neck in early stages.[2] [3]
Post-cricoid carcinoma is rare and seen in certain high-risk groups such as patients with the Plummer–Vinson syndrome. This subtype tends to be commoner in females. These lesions show submucosal spread, most commonly toward the cervical esophagus. Due to the submucosal spread, its true extent becomes more apparent with axial or sagittal MR images.[2] [3] [Figure 13] shows an example of post cricoid carcinoma.
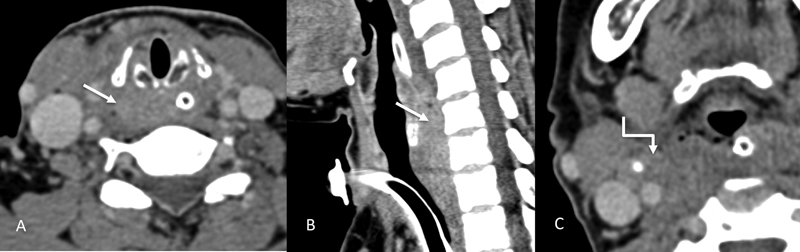
| Figure 13:Imaging of post cricoid cancer. Axial and sagittal CT images (A–C) showing a mass (straight arrows) involving the post cricoid region and a metastatic right level II node (shouldered arrow).
Posterior pharyngeal wall carcinoma commonly involves the oropharynx too. These appear as asymmetrical thickening of the posterior pharyngeal wall. Prevertebral space involvement is rare and can be reliably excluded on CT and MRI by observing the retropharyngeal fat plane.[2] [3]
A short axis diameter of at least 10 mm, round shape, presence of necrosis (irrespective of size) and extranodal extension (node showing indistinct spiculated margins) are the general criteria to detect involved nodes on CT and MRI.[1] [2]
The most common site of distant metastasis is lung. Bone and liver are the other frequent sites.[1] [2]
|
T staging |
|
|---|---|
|
Supraglottic SCC |
|
|
T1 |
Tumor confined to one supraglottic subsite with normal vocal cord mobility |
|
T2 |
Tumor involving mucosa of more than one supraglottic subsite, with no cord fixation |
|
T3 |
Tumor limited to the larynx, with vocal cord fixation and/or invasion of post cricoid region or pre-epiglottic space |
|
T4A |
Tumor invading through cricoid cartilage and/ or other extra-laryngeal tissues (trachea, cervical soft tissue, strap muscle, thyroid, esophagus) (resectable) |
|
T4B |
Tumor invading prevertebral space, encasing carotid artery or invading mediastinal structures (unresectable) |
|
Glottic SCC |
|
|
T1 |
Tumor confined to vocal cord(s) with normal mobility (may involve anterior or posterior commissure) T1A-Limited to one cord T1B-Involving both cords |
|
T2 |
Tumor extending to supra and/or subglottis with impaired vocal cord mobility |
|
T3 |
Tumor limited to larynx, with vocal cord fixation and/or invasion of paraglottic space and/ or inner cortex of thyroid cartilage |
|
T4A |
Tumor invading through thyroid cartilage and/ or other extra-laryngeal tissues (trachea, cervical soft tissue, strap muscle, thyroid, esophagus, deep extrinsic muscles of tongue) (resectable) |
|
T4B |
Tumor invading prevertebral space, encasing carotid artery or invading mediastinal structures (very advanced local disease) |
|
Subglottic SCC |
|
|
T1 |
Tumor confined to subgottis |
|
T2 |
Tumor extending to vocal cord(s) with normal or impaired mobility |
|
T3 |
Tumor limited to larynx with vocal cord fixation |
|
T4A |
Tumor invading through cricoid and/or thyroid cartilage and/or other extralaryngeal tissues (trachea, cervical soft tissue, strap muscle, thyroid, esophagus, deep extrinsic muscles of tongue) (resectable) |
|
T4B |
Tumor invading prevertebral space, encasing carotid artery or invading mediastinal structures (Unresectable) |
|
Hypopharyngeal SCC |
|
|
T1 |
Tumor limited to one subsite of hypopharynx and/or ≤ 2 cm in greatest dimension |
|
T2 |
Tumor extends into adjacent subsite of hypopharynx or adjacent site (larynx, oropharynx) and/or > 2 cm but ≤ 4 cm without fixation of hemilarynx |
|
T3 |
Tumor > 4 cm, or clinical fixation of hemilarynx, or extension to esophageal mucosa |
|
T4a |
Tumor invades thyroid cartilage and/or cricoid cartilage and/or hyoid bone and/or thyroid gland and/or esophageal muscle and/or central compartment soft tissue (prelaryngeal strap muscles and subcutaneous fat) (moderately advanced local disease) |
|
T4b |
Tumor invading prevertebral space, encasing carotid artery or invading mediastinal structures (very advanced local disease) |
|
N staging |
|
|
Nx |
Regional nodes cannot be assessed |
|
N0 |
No regional nodal metastasis |
|
N1 |
Metastasis in single ipsilateral lymph node ≤ 3 cm in greatest dimension with no extranodal extension (ENE) |
|
N2a |
Metastasis in single ipsilateral lymph node > 3 cm but ≤ 6 cm in greatest dimension with no ENE; or single ipsilateral lymph node up to 3 cm in the greatest dimension with ENE |
|
N2b |
Metastasis in multiple ipsilateral lymph nodes, ≤ 6 cm in greatest dimension with no ENE |
|
N2c |
Metastasis in bilateral or contralateral lymph nodes, ≤ 6 cm in greatest dimension with no ENE |
|
N3a |
Metastasis in a lymph node > 6 cm in greatest dimension with no ENE |
|
N3b |
Metastasis in a single ipsilateral lymph node > 3 cm with ENE; or multiple ipsilateral, contralateral or bilateral nodes with ENE; or single contralateral node with ENE |
|
M staging |
|
|
M0 |
No distant metastasis |
|
M1 |
Distant metastasis |

| Figure 14:Pre-chemotherapy (A and C) and post chemotherapy (B and D) imaging of the previously shown pyriform sinus cancer. Post chemotherapy axial CT images (B and D) showing near complete resolution of the primary mass and the metastatic bilateral level II nodes (shouldered arrows).
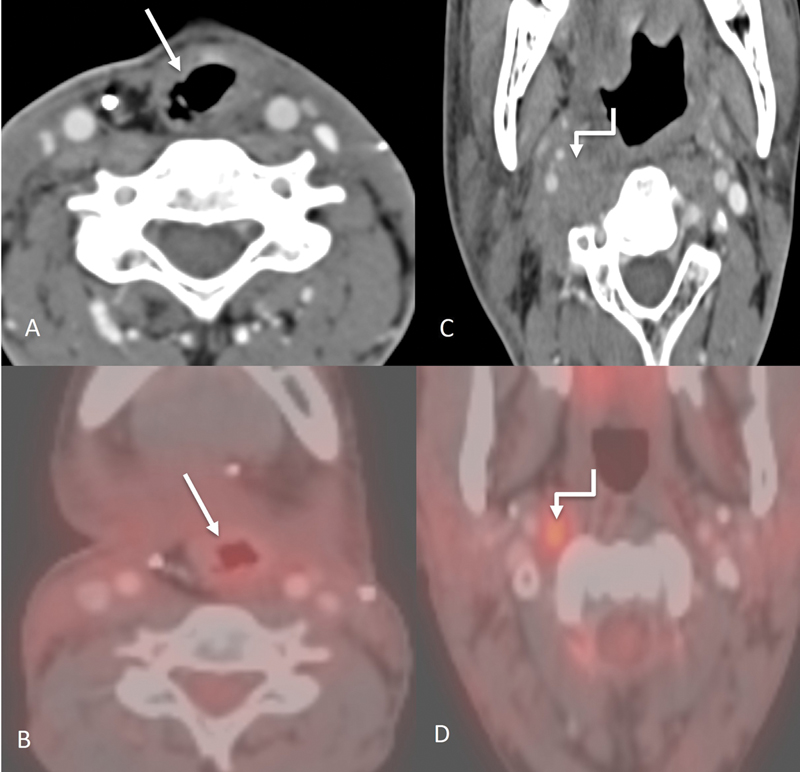
| Figure 15:Post treatment imaging. Axial CT image (A) of a post laryngectomy patient showing the altered anatomy postsurgery (straight arrow) with a corresponding PET CT image (B) showing no significant uptake to suggest recurrence at the locoregional site. However, an enlarged right retropharyngeal node is seen on axial CT image (C), (marked with shouldered arrow) which also showed uptake on the corresponding PET CT image (D).
As per the NCCN guidelines in oncology for head and neck cancers,[22] a clinical assessment is to be done 4 to 8 weeks after chemotherapy or radiotherapy. If there is response clinically, a CT and/or MRI of the primary site and the neck should be performed within 8 to 12 weeks or an FDG PET/CT should be performed within 12 weeks to assess the extent of the disease. If there is residual primary tumor or disease progression clinically, a CT and/or an MRI within 4 to 8 weeks or an FDG PET/CT should be performed.[23]
Surgery causes significant distortion of the anatomy, thus the diagnosis of recurrence becomes challenging. Differentiating disease from postoperative change is difficult in view of redundant mucosa, obliteration of the paralaryngeal fat planes during surgery or postoperative granuloma. Recurrence post-surgery is generally seen as focal nodular areas or soft tissue at the surgical site. Obvious cartilage destruction and soft tissue masses greater than 1 cm in size are suggestive of recurrent tumor. Recurrence is commonly seen at the cut margins of the surgery where the tumor was previously located.[16] [20]
Radiation therapy, if successful, decreases the tumor volume within 4 months. In case, at least 50%-of the mass is visible 4 months after radiation, treatment failure should be considered. Radiation therapy produces edema of the laryngopharynx, thickening of the epiglottis, arytenoids, and aryepiglottic folds with abnormal enhancement. The normal fat appears stippled. These appearances may be marked and persistent beyond 6 months.[16] [20] [Figure 16] shows typical post radiation therapy changes.
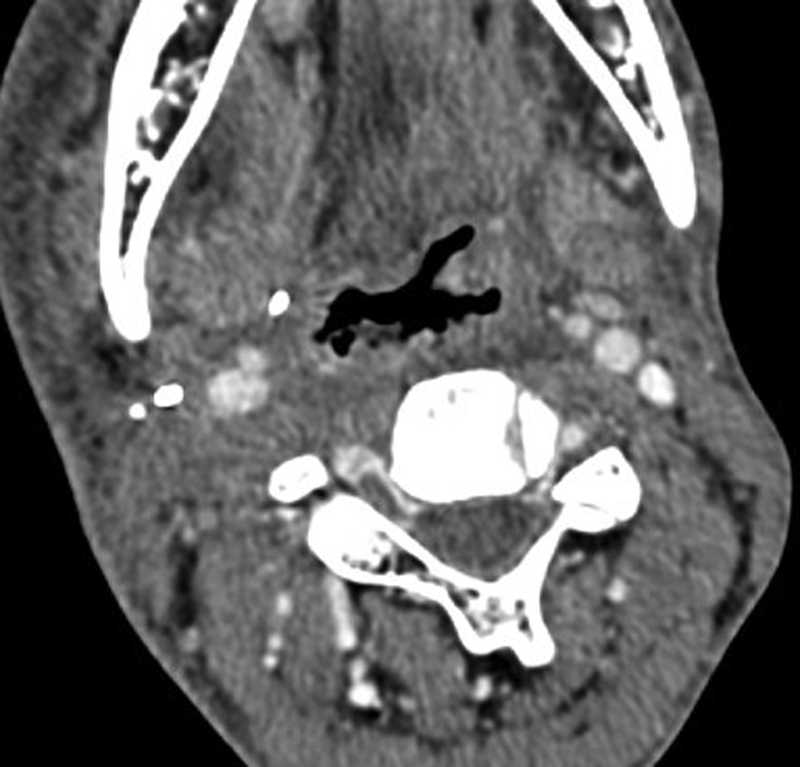
| Figure 16:Post treatment imaging. Axial CT image showing the changes caused by radiation therapy in the form of diffuse mucosal edematous changes and thickening of the skin and subcutaneous soft tissues.
Radiation-induced chondronecrosis causes fragmentation, sclerosis and/or lysis of the cartilages. Differentiating this from recurrence is again a dilemma because both occur within a year of radiotherapy.
Knowledge of post-treatment appearances on CT and MRI scans is important to avoid unnecessary biopsies. PET-CT has more diagnostic accuracy in detecting tumor recurrence, especially when done 2 to 3 months after the completion of treatment.[16] [20]
Carcinogens have been known to induce premalignant changes at more than one site in the aerodigestive tract, resulting in multiple primary tumors or secondary primary tumors, a concept known as ‘field cancerization’[24]. Proposed mechanisms for this include multiple genetic aberrations, or alternatively, migration of transformed clonal cells.[24] CT, MRI, and FDG PET-CT play an important role in detecting multiple primary tumors and second primary tumors at a distant site.[25] This prevents unnecessary radical treatment of the tumor. Other methods to detect multiple/second primary tumors include fluorescence visualization.[25] Chemopreventive agents such as 13-cis retinoic acid are used to treat and prevent field cancerization.[24]
Principles of Management
Early stage I-II is treated with single modality either radiotherapy or surgery. Advanced disease with stage III-IV is treated with multimodality of either chemoradiotherapy or surgery followed by adjuvant radiotherapy with or without concurrent chemotherapy.[12] The treatment of choice for functional larynx with T1-T3 disease is organ preservation protocol of chemoradiotherapy (VA trial, RTOG 9111).[26] In select young patients with good performance status conservation, laryngeal surgery can be considered if surgical expertise is present. Early laryngeal cancers can also be treated with transoral laser surgery. Total laryngectomy is the treatment of choice in locally advanced T4 disease along with bilateral neck dissection[27] Positive node, perineural invasion, lymphatic invasion, vascular embolism, pT3 or pT4 primary disease are indications for adjuvant radiotherapy, which should be supplemented with chemotherapy in cases with extranodal extension and positive margins.[27]
In cases of carcinoma of the hypopharynx, early disease is treated with radiotherapy alone. Partial laryngectomy or transoral laser surgery can be offered in select cases. Locally advanced disease without exolaryngeal spread or cartilage erosion is treated with either chemoradiotherapy or neoadjuvant chemotherapy followed by chemoradiotherapy in responders (EORTC 24891).[28] For disease with exolaryngeal spread/cartilage erosion (T4 disease) and/or a dysfunctional larynx total laryngopharyngectomy with bilateral neck dissection should be offered. The indications of adjuvant treatment are same as mentioned above.
Patients with distant metastasis are treated with palliative intent for symptomatic relief. Treatment for the metastatic disease depends on the performance status of the patient. If good, systemic therapy is considered and if the performance status is adverse, best supportive care is advised.[12]
Follow-up Imaging and Management of Recurrent Disease including Specific Interventional and Palliative Measures
In cases of resectable locoregional recurrence, these patients should be offered surgery. If any adverse feature is identified on histopathology, adjuvant treatment with chemotherapy/RT should be considered. If the locoregional recurrence is not resectable, then these patients should be treated with systemic therapy/radiotherapy. Treatment for the metastatic recurrent disease depends on the performance status of the patient. Systemic therapy is considered if it is good and if the performance status is poor, the best supportive care is advised.[12]
Summary of Recommendations
CT is generally used for assessing the primary disease, with MRI being used in doubtful cases especially for cartilage erosion.
Ultrasound is useful for assessing neck nodes and also guide their fine needle aspiration.
CT chest is used to rule out lung metastasis in advanced cases of laryngeal cancer; FDG PET-CT is used for distant metastases in cases with high locoregional burden and for detection of lymph node metastatic disease.
Response assessment should ideally be done with the modality with which the baseline evaluation was done.
Recurrence can be detected with CT or MRI or FDG PET-CT.
Conflict of Interest
None declared.
Note
The article is not under consideration for publication elsewhere. Each author participated sufficiently for the work to be submitted. Publication is approved by all authors.
References
- Joshi VM, Wadhwa V, Mukherji SK. Imaging in laryngeal cancers. Indian J Radiol Imaging 2012; 22 (03) 209-226
- Hermans R. Staging of laryngeal and hypopharyngeal cancer: value of imaging studies. Eur Radiol 2006; 16 (11) 2386-2400
- Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol 2008; 66 (03) 460-479
- Hashibe M, Boffetta P, Zaridze D. et al. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol 2007; 165 (07) 814-820
- Bobdey S, Jain A, Balasubramanium G. Epidemiological review of laryngeal cancer: An Indian perspective. Indian J Med Paediatr Oncol 2015; 36 (03) 154-160
- Bradley PJ. Epidemiology of hypopharyngeal cancer. In: Hypopharyngeal Cancer . Vol. 18. Basel, Switzerland: Karger Publishers; 2019: 1-14
- Yang CY, Andersen PE, Everts EC, Cohen JI. Nodal disease in purely glottic carcinoma: is elective neck treatment worthwhile?. Laryngoscope 1998; 108 (07) 1006-1008
- Spector GJ. Distant metastases from laryngeal and hypopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec 2001; 63 (04) 224-228
- Yücel OT, Yilmaz T, Unal OF, Turan E. Distant metastases in laryngeal squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research. CR (East Lansing Mich) 1999; 18 (03) 285-288
- Liu WW, Zeng ZY, Guo ZM, Xu GP, Yang AK, Zhang Q. Distant metastases and their significant indicators in laryngeal cancer [article in Chinese]. Zhonghua Er Bi Yan Hou Ke Za Zhi 2003; 38 (03) 221-224
- Babu G, Prabhash K, Chaturvedi P. et al. Indian clinical practice consensus guidelines for the management of hypopharyngeal cancer. Indian J Cancer 2020; 57 (Supplement): S16-S18
- Network NCC. NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancer version 1. 2021
- Wu JH, Zhao J, Li ZH. et al. Comparison of CT and MRI in diagnosis of laryngeal carcinoma with anterior vocal commissure involvement. Sci Rep 2016; 6 (01) 30353
- Olliff J, Richards P, Connor S, Wong WL, Beale T, Madani G. Head and neck cancers. In: Nicholson T. ed. Recommendations for Cross-Sectional Imaging in Cancer Management. 2nd ed.. London: The Royal College of Radiologists; 2014: 3-19
- Collins SR. Direct and indirect laryngoscopy: equipment and techniques. Respir Care 2014; 59 (06) 850-862 , discussion 862–864
- Connor S. Laryngeal cancer: how does the radiologist help?. Cancer Imaging 2007; 7 (01) 93-103
- Zhang SC, Zhou SH, Shang DS, Bao YY, Ruan LX, Wu TT. The diagnostic role of diffusion-weighted magnetic resonance imaging in hypopharyngeal carcinoma. Oncol Lett 2018; 15 (04) 5533-5544
- Alaa K. Nagham N, Mohamed DG, Sherif M. Role of magnetic resonant imaging in laryngeal cancer. The Medical Journal of Cairo University 2018; 86 (June): 1683-1692
- Xia CX, Zhu Q, Zhao HX, Yan F, Li SL, Zhang SM. Usefulness of ultrasonography in assessment of laryngeal carcinoma. Br J Radiol 2013; 86 (1030): 20130343
- Hermans R. Post-treatment imaging of head and neck cancer. Cancer Imaging 2004; 4 Spec No A: S6-S15
- Mehanna H, Wong WL, McConkey CC. et al; PET-NECK Trial Management Group. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016; 374 (15) 1444-1454
- Colevas AD, Yom SS, Pfister DG. et al. NCCN guidelines insights: head and neck cancers, Version 1.2018. J Natl Compr Canc Netw 2018; 16 (05) 479-490
- Seebauer CT, Hackenberg B, Grosse J. et al. Routine restaging after primary non-surgical treatment of laryngeal squamous cell carcinoma-a review. Strahlenther Onkol 2021; 197 (03) 167-176
- Aparna M, Shenai P, Chatra L. et al. Field cancerization: a review. Arch Med Health Sci 2013; 1: 136-139
- Raviraj J. Oral field cancerization: a review. J Indian Acad Oral Med Radiol 2010; 22: 201-205
- Mannelli G, Lazio MS, Luparello P, Gallo O. Conservative treatment for advanced T3-T4 laryngeal cancer: meta-analysis of key oncological outcomes. Eur Arch Otorhinolaryngol 2018; 275 (01) 27-38
- Forastiere AA, Zhang Q, Weber RS. et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013; 31 (07) 845-852
- Lefebvre JL, Andry
G, Chevalier D. et al; EORTC Head and Neck Cancer
Group. Laryngeal preservation
with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891.
Ann Oncol 2012; 23 (10) 2708-2714
Address for correspondence
Arpita Sahu, MDRoom No. 119, Main Building, Tata Memorial Hospital, Parel, Mumbai, MaharashtraIndiaEmail: drarpitasahu@gmail.comPublication History
Article published online:
24 January 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Normal laryngeal anatomy. Coronal CT image (A) showing normal anatomy of the larynx and its subdivisions (supraglottis between blue and white lines; glottis between white and green lines; subglottis between the green and orange lines). Normal appearance of the thyroid cartilage (straight arrow labeled T) and cricoid cartilage (straight arrow) shown on image (B) and on axial T2W MRI image (C).

| Figure 2:Normal anatomy of the hypopharynx. Figure showing normal appearance of the hypopharynx (box) on sagittal CT image (A) and of the post cricoid region (arrow) on the axial CT image (B).

| Figure 3:Ultrasound image showing a round neck node with loss of fatty hilum, suggestive of an involved node.

| Figure 4:Supraglottic laryngeal cancer. Axial and coronal CT images (A, C) showing a heterogeneously enhancing lesion (straight arrows) involving the left false vocal cord. The true vocal cords are normal in appearance and not involved as shown by the other axial image (B).

| Figure 5:Supraglottic laryngeal cancer. Axial, coronal, and sagittal CT images (A–C) showing the proliferative irregular mass (straight arrows) involving the right vallecula and supra and infrahyoid epiglottis on the right with involvement of the median glosso-epiglottic fold. There is involvement of the pre-epiglottic space also (blue arrowhead). Sagittal left paramedian CT image (D) showing normal uninvolved epiglottis on the left (shouldered arrow).

| Figure 6:Glottic laryngeal cancer. Axial and coronal CT images (A and B) showing a proliferative lesion (straight arrows) involving the true vocal cords bilaterally, with almost complete obliteration of the airway, necessitating a tracheostomy. The inner cortex of the right thyroid cartilage lamina is also involved (shouldered arrow) by the mass. No suspicious neck nodes were seen (C), which is usually the case in glottis cancers.

| Figure 7:Subglottic involvement. Axial and coronal CT images (A and B) showing subglottic involvement (straight arrows) of the laryngeal mass.

| Figure 8:Transglottic cancer involving supraglottis and glottis. Axial and coronal CT images (A–C) showing an enhancing exophytic mass (straight arrows) involving the right aryepiglottic fold and right vocal cord, with involvement of the right paraglottic space. Extralaryngeal spread of disease is seen (yellow arrow in B). An enlarged metastatic necrotic right level II node (shouldered arrow) is also seen (D).

| Figure 9:Transglottic laryngeal cancer. Axial CT images (A–C) showing the primary enhancing mass (straight arrows) involving the true vocal cords bilaterally and left false cord. There is gross involvement of the thyroid cartilage (more on the left side) with subtle extralaryngeal spread anteriorly (shouldered arrows). Right arytenoid cartilage appears sclerosed. The left para glottic space is also involved. In addition, lung nodules and liver lesions were also identified (D–F).

| Figure 10:Transglottic cancer. Axial T1W (A), T2W (B), post contrast T1W (C), coronal STIR (D), sagittal T2W (E) images showing the T1 isointense, T2 intermediate intensity, STIR hyperintense heterogeneously enhancing mass (arrows) involving the supraglottis and glottis. The mass shows restricted diffusion as seen on axial DWI and ADC images (F and G).

| Figure 11:Cartilage involvement-axial CT shows an enhancing mass (*) involving the right true cord, invading the thyroid cartilage on the right side (white arrow) and encasing the right arytenoid cartilage (yellow arrow).

| Figure 12:Hypopharyngeal carcinoma involving pyriform sinus. Axial and coronal CT images (A, B) showing a proliferative mass (straight arrows) involving the left pyriform sinus. Enlarged metastatic bilateral level II nodes (shouldered arrow) are also seen on the axial CT image (C). Corresponding PET CT images (D and E) show significant uptake in the primary mass and metastatic neck nodes.

| Figure 13:Imaging of post cricoid cancer. Axial and sagittal CT images (A–C) showing a mass (straight arrows) involving the post cricoid region and a metastatic right level II node (shouldered arrow).

| Figure 14:Pre-chemotherapy (A and C) and post chemotherapy (B and D) imaging of the previously shown pyriform sinus cancer. Post chemotherapy axial CT images (B and D) showing near complete resolution of the primary mass and the metastatic bilateral level II nodes (shouldered arrows).

| Figure 15:Post treatment imaging. Axial CT image (A) of a post laryngectomy patient showing the altered anatomy postsurgery (straight arrow) with a corresponding PET CT image (B) showing no significant uptake to suggest recurrence at the locoregional site. However, an enlarged right retropharyngeal node is seen on axial CT image (C), (marked with shouldered arrow) which also showed uptake on the corresponding PET CT image (D).

| Figure 16:Post treatment imaging. Axial CT image showing the changes caused by radiation therapy in the form of diffuse mucosal edematous changes and thickening of the skin and subcutaneous soft tissues.
References
- Joshi VM, Wadhwa V, Mukherji SK. Imaging in laryngeal cancers. Indian J Radiol Imaging 2012; 22 (03) 209-226
- Hermans R. Staging of laryngeal and hypopharyngeal cancer: value of imaging studies. Eur Radiol 2006; 16 (11) 2386-2400
- Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol 2008; 66 (03) 460-479
- Hashibe M, Boffetta P, Zaridze D. et al. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol 2007; 165 (07) 814-820
- Bobdey S, Jain A, Balasubramanium G. Epidemiological review of laryngeal cancer: An Indian perspective. Indian J Med Paediatr Oncol 2015; 36 (03) 154-160
- Bradley PJ. Epidemiology of hypopharyngeal cancer. In: Hypopharyngeal Cancer . Vol. 18. Basel, Switzerland: Karger Publishers; 2019: 1-14
- Yang CY, Andersen PE, Everts EC, Cohen JI. Nodal disease in purely glottic carcinoma: is elective neck treatment worthwhile?. Laryngoscope 1998; 108 (07) 1006-1008
- Spector GJ. Distant metastases from laryngeal and hypopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec 2001; 63 (04) 224-228
- Yücel OT, Yilmaz T, Unal OF, Turan E. Distant metastases in laryngeal squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research. CR (East Lansing Mich) 1999; 18 (03) 285-288
- Liu WW, Zeng ZY, Guo ZM, Xu GP, Yang AK, Zhang Q. Distant metastases and their significant indicators in laryngeal cancer [article in Chinese]. Zhonghua Er Bi Yan Hou Ke Za Zhi 2003; 38 (03) 221-224
- Babu G, Prabhash K, Chaturvedi P. et al. Indian clinical practice consensus guidelines for the management of hypopharyngeal cancer. Indian J Cancer 2020; 57 (Supplement): S16-S18
- Network NCC. NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancer version 1. 2021
- Wu JH, Zhao J, Li ZH. et al. Comparison of CT and MRI in diagnosis of laryngeal carcinoma with anterior vocal commissure involvement. Sci Rep 2016; 6 (01) 30353
- Olliff J, Richards P, Connor S, Wong WL, Beale T, Madani G. Head and neck cancers. In: Nicholson T. ed. Recommendations for Cross-Sectional Imaging in Cancer Management. 2nd ed.. London: The Royal College of Radiologists; 2014: 3-19
- Collins SR. Direct and indirect laryngoscopy: equipment and techniques. Respir Care 2014; 59 (06) 850-862 , discussion 862–864
- Connor S. Laryngeal cancer: how does the radiologist help?. Cancer Imaging 2007; 7 (01) 93-103
- Zhang SC, Zhou SH, Shang DS, Bao YY, Ruan LX, Wu TT. The diagnostic role of diffusion-weighted magnetic resonance imaging in hypopharyngeal carcinoma. Oncol Lett 2018; 15 (04) 5533-5544
- Alaa K. Nagham N, Mohamed DG, Sherif M. Role of magnetic resonant imaging in laryngeal cancer. The Medical Journal of Cairo University 2018; 86 (June): 1683-1692
- Xia CX, Zhu Q, Zhao HX, Yan F, Li SL, Zhang SM. Usefulness of ultrasonography in assessment of laryngeal carcinoma. Br J Radiol 2013; 86 (1030): 20130343
- Hermans R. Post-treatment imaging of head and neck cancer. Cancer Imaging 2004; 4 Spec No A: S6-S15
- Mehanna H, Wong WL, McConkey CC. et al; PET-NECK Trial Management Group. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016; 374 (15) 1444-1454
- Colevas AD, Yom SS, Pfister DG. et al. NCCN guidelines insights: head and neck cancers, Version 1.2018. J Natl Compr Canc Netw 2018; 16 (05) 479-490
- Seebauer CT, Hackenberg B, Grosse J. et al. Routine restaging after primary non-surgical treatment of laryngeal squamous cell carcinoma-a review. Strahlenther Onkol 2021; 197 (03) 167-176
- Aparna M, Shenai P, Chatra L. et al. Field cancerization: a review. Arch Med Health Sci 2013; 1: 136-139
- Raviraj J. Oral field cancerization: a review. J Indian Acad Oral Med Radiol 2010; 22: 201-205
- Mannelli G, Lazio MS, Luparello P, Gallo O. Conservative treatment for advanced T3-T4 laryngeal cancer: meta-analysis of key oncological outcomes. Eur Arch Otorhinolaryngol 2018; 275 (01) 27-38
- Forastiere AA, Zhang Q, Weber RS. et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013; 31 (07) 845-852
- Lefebvre JL, Andry G, Chevalier D. et al; EORTC Head and Neck Cancer Group. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol 2012; 23 (10) 2708-2714


 PDF
PDF  Views
Views  Share
Share

