Imaging Recommendations for Diagnosis, Staging, and Management of Oral Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 150-158
DOI: DOI: 10.1055/s-0042-1760314
Abstract
Oral cavity cancers contribute to a majority of cancers in India. Clinical examination alone cannot determine the deeper extent of the disease; therefore, need for cross-sectional imaging including computed tomography and magnetic resonance imaging becomes indispensable for pre-treatment evaluation to decide optimal plan of management. Oral cavity squamous cell cancers (OSCC) can be treated with surgery alone, whereas deep muscle, neurovascular, osseous, or nodal involvement on imaging suggests advanced disease that requires a combination of surgery, radiation, and/or chemotherapy. Because of the complex anatomy of the oral cavity and its surrounding structures, imaging is crucial for locoregional staging and early detection of distant metastases. Imaging plays indispensable role not only in diagnosis but also in planning the management. An optimal guideline paper for developing countries like India is lacking that not only helps standardize the management but will also assist oncologists make reasonable decisions and reduce the unnecessary imaging. This imaging guideline paper will discuss the optimal imaging in diagnosis and management OSCC for Indian subcontinent.
Keywords
computed tomography - guidelines - magnetic resonance imaging - oral cancer - imagingThe article is not under consideration for publication elsewhere. Each author participated sufficiently for the work to be submitted. Publication is approved by all authors.
Supplementary MaterialPublication History
Article published online:
01 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Oral cavity cancers contribute to a majority of cancers in India. Clinical examination alone cannot determine the deeper extent of the disease; therefore, need for cross-sectional imaging including computed tomography and magnetic resonance imaging becomes indispensable for pre-treatment evaluation to decide optimal plan of management. Oral cavity squamous cell cancers (OSCC) can be treated with surgery alone, whereas deep muscle, neurovascular, osseous, or nodal involvement on imaging suggests advanced disease that requires a combination of surgery, radiation, and/or chemotherapy. Because of the complex anatomy of the oral cavity and its surrounding structures, imaging is crucial for locoregional staging and early detection of distant metastases. Imaging plays indispensable role not only in diagnosis but also in planning the management. An optimal guideline paper for developing countries like India is lacking that not only helps standardize the management but will also assist oncologists make reasonable decisions and reduce the unnecessary imaging. This imaging guideline paper will discuss the optimal imaging in diagnosis and management OSCC for Indian subcontinent.
Keywords
computed tomography - guidelines - magnetic resonance imaging - oral cancer - imagingIntroduction
Head and neck cancers are the sixth most common cancer worldwide, with oral cavity squamous cell carcinoma (OSCC) being the most common and having the high morbidity and fatality rates.[1] For proper management, timely diagnosis and correct tumor staging are vital. Radiologic imaging is routinely used to assess the disease extension in supplementation with clinical examination. The most common histology is SCC, which accounts for the vast majority of oral cancers.[2] The symptoms of malignancy, the methods by which it spreads, and the prognosis are all highly variable, and are largely determined by the anatomic region where the initial tumor develops. For diagnostic assessment and appropriate treatment planning, it is of utmost importance to understand the oral anatomy and most typical pathways of dissemination of OSCC.[3]
Risk Factors and Etiopathogenesis
Risk factors for OSCC include quid chewing, poor oral hygiene, tobacco, alcohol consumption, and sharp tooth/denture.[4] [5] The World Health Organization describes the oral potentially malignant disorders that may transform into carcinoma later in life. These include leukoplakia, erythroplakia, erythroleukoplakia, oral submucous fibrosis, smokeless tobacco keratosis, lichen planus, and discoid lupus erythematosus.[6] The morphological spectrum of oral potentially malignant disorders varies from acanthosis, hyperkeratosis, to dysplasia and carcinoma in situ.[7]
Epidemiology and Clinical Presentation in India and Global
Oral cancer constitutes sixth most frequent malignancies in Asia with approximately 274,300 new cases occurring each year.[8] Age standardized incidence rate (ASIR) in Sri Lanka, Taiwan, Bangladesh, India, and Pakistan, is far more than the world ASIR (10.5 for men and 4.02 for women). In India, ASIR of 12.7/100,000 in men (Bombay) and 10.0/100,000 in women (Bangalore) has been reported.[9] The plausible reason is the rampant use of chewed tobacco and common custom of chewing beetle quid containing areca nut along with slaked lime. Patients usually present with nonhealing ulcer, pain, bleeding, poorly fitting dentures, speech alteration, and neck lymph nodes.[10] Examination includes inspection of the oral cavity along with palpation of the lesion under anesthesia to assess the submucosal extent of disease. The neck is thoroughly palpated to detect lymph node metastasis that is large in size, hard in consistency, and may be fixed to surrounding structures.[11] The upper aerodigestive tract should be examined for any synchronous second primary. Biopsy of tumor and/or lymph nodes is done to establish the diagnosis and further workup is planned after histological confirmation.
Imaging Referral Guidelines
The American Joint Committee on Cancer/International Union Against Cancer staging method is a tool that allows physicians all over the globe to stage cancer before any therapy, after surgical resection, and at the time of recurrence.[12] Staging divides patients into prognostic groups, making it easy to choose the best treatment strategy, schedule treatment, and predict prognosis based on the stage of the disease. Updates in the 8th edition are as shown in [Table 1].[13]
|
Seventh edition |
Eighth edition |
|
|---|---|---|
|
T1 |
Tumor < 2 cm |
Tumor ≤ 2 cm, ≤ 5 mm depth of invasion |
|
T2 |
Tumor 2–4 cm |
Tumor ≤ 2 cm, >5 mm and ≥ 10 mm depth of invasion or tumor > 2 cm but ≤ 4 cm and depth of invasion ≤ 10 mm |
|
T3 |
Tumor > 4 cm |
Tumor > 4 cm and depth of invasion < 10 mm or tumor <4cm>10 mm |
|
T4a |
Moderately advanced local disease Lip: tumor invades through the cortical bone or involves inferior alveolar nerve, floor of mouth, or skin of face Oral cavity: tumor involves adjacent structures such as cortical bone of maxilla or mandible, maxillary sinus or skin of face, or extrinsic muscles of tongue |
Extrinsic muscles of tongue removed, included extensive tumors with bilateral tongue involvement or tumor > 4cm and depth of invasion > 10mm |
|
T4b |
Very advanced local disease; tumor invades masticator space, pterygoid plates, skull base and/or encases the internal carotid artery |
No change |
|
N1 |
Metastases to single lymph node, 3 cm or less in greatest dimension |
Same, except node must be extranodal extension negative |
|
N2a |
Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension |
Same, except node must be extranodal extension negative |
|
N2b |
Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension |
Same, except nodes must be extranodal extension negative |
|
N2c |
Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension |
Same, except nodes must be extranodal extension negative |
|
N3 |
Metastases to node > 6 cm |
Subdivided into 3a: Same as N3 before, but extranodal extension negative 3b: any node with extranodal extension |
|
Imaging setting |
Preferred imaging modality |
|---|---|
|
Screening |
CECT or CE-MRI[a] (no proven role) |
|
Diagnosis: |
|
|
a) Diagnostic |
For gingivobuccal cancer—CECT PNS and thorax For tongue carcinoma—CE-MRI[a] plus NCCT thorax FDG-PET-CT |
|
b) Intervention |
CT-guided biopsy/FNAC for deep-seated lesions USG-guided biopsy/ FNAC for nodes and superficially seated lesions |
|
Management |
|
|
a) Post-surgery |
CECT/ CE-MRI[a] (OR) FDG-PET-CT |
|
b) Neoadjuvant, adjuvant or palliative chemotherapy |
CECT/CE-MRI[a] (OR) FDG-PET-CT |
|
Follow-up |
FDG-PET-CT (OR) CECT/CE-MRI[a] |
|
Parameter |
Characteristics |
|---|---|
|
Scanner type |
Helical scanner |
|
Slice thickness |
0.75 mm |
|
Intravenous contrast |
Iodine-based contrast agent at 3 to 5mL/sec flow rate (total volume—80 mL) |
|
Maneuvers |
Puffed-cheek technique |
|
Acquisition time |
40–50 seconds |
|
Reconstruction |
Bone algorithm reconstruction Multiplanar reconstruction in coronal plane or parasagittal plane |
|
Anatomical coverage |
Pituitary fossa to arch of aorta. Include thorax and upper abdomen in venous phase—staging |
|
Sequence |
Plane of acquisition |
Slice thickness |
|---|---|---|
|
T1W FS-FSE |
Coronal |
4 mm |
|
STIR |
Coronal |
4 mm |
|
T2W FS-FSE |
Sagittal |
4 mm |
|
T2W FS-FSE |
Axial |
4 mm |
|
T1W FS-FSE |
Axial |
4 mm |
|
DWI |
Axial |
4 mm |
|
Postgadolinium contrast T1W FS-FSE |
Axial, coronal, and sagittal planes |
4 mm |
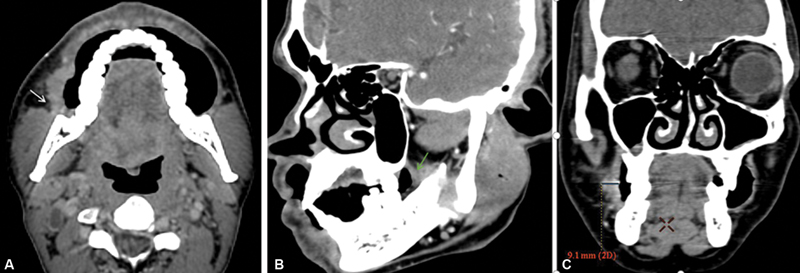
| Figure 1:(A) Axial section shows an ill-defined thickening involving right buccal mucosa buccinator complex with loss of fat planes with masseter muscle (white arrow). (B) Sagittal oblique shows an ill-defined thickening involving retromolar trigone region (green arrow) highlighting importance of oblique reformation. (C) Coronal reformatted images show technique to measure depth of invasion.
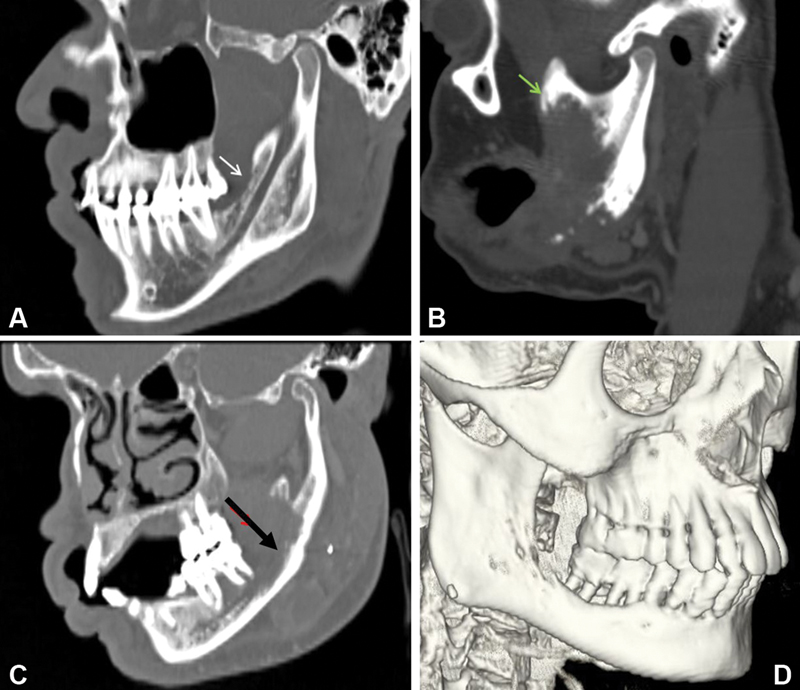
| Figure 2:Oblique sagittal reformatted images showing superficial cortical erosion (white arrow) of the mandible, (B) erosion of the coronoid process of the mandible (Green arrow), (C) cortical and medullary erosion with involvement of inferior alveolar canal (black arrow) of mandible. (D) Shaded surface display image shows erosion of mandible and important in planning surgical reconstruction.
Tongue: CE-MRI has better soft tissue resolution and hence it is the modality of choice for evaluation of tongue. MRI better defines the DOI, muscles of tongue involvement, midline extension, FOM, neurovascular structure, and posterior third tongue involvement.[27] However, for the evaluation of mandibular cortical bone involvement CECT is preferred to MRI.
Artefacts: Both CT and MRI images are susceptible to artefacts in the presence of dental implants or amalgams. However, CT has its advantage in this aspect, as it permits artefact reduction via employment of tube angulation and algorithms like metal artefact reduction.[18]
Interventions: Oral cavity lesions are usually sampled per orally in clinical setting; however, image guidance is required if the lesion is deep seated as in masticator space, ITF, parapharyngeal or retropharyngeal space. Generally, CT-guided sampling is preferred in these deep-seated subsites.[28] FDG-PET-CT has major advantage in guiding the sampling to the site of FDG avidity. USG can be employed for sampling of superficial seated lesions, lymph nodes, etc.
Staging
The role of imaging in staging relies on identifying locoregional extent and nodal and distant metastases. CECT whenever performed for initial workup should include thorax and upper abdomen as part of staging evaluation. Involvement of the ITF, masticator space, and presence of perineural spread are important predictors of locoregional staging. Perineural spread ([Supplementary Table S2]) of disease can be identified as thickening, enhancement of nerve, and widening of neural foramina as shown in [Tables 5] and [6].[18] [29] [30] Even though CECT can detect the presence of perineural spread, MRI is more sensitive as it can depict even subtle perineural spread. The most commonly involved nerve being mandibular in gingivobuccal cancer and maxillary division of trigeminal nerve in carcinoma hard palate is shown in [Figs. 3] and [4]. ITF involvement ([Supplementary Table S3]) can be subdivided as high and low ITF, based on the presence of disease involvement above or below the level of the sigmoid notch of mandible.[18] [31] [32] [33] [34] Regional metastasis is common to the cervical lymph nodes. The frequent sites of distant metastases in oral cavity cancers are lungs, liver, bones, and mediastinal nodes. Cervical lymph node metastases can be detected by USG as it better depicts the morphology, shape, presence of cystic change, and nature of fatty hilum. Also, USG guidance can be used for sampling of these nodes for FNAC or biopsy. CECT and CE-MRI both equally depict the extranodal extension (ENE), an important prognostic marker in predicting advanced nodal disease and local recurrence and are considered the best imaging modality for nodal metastases ([Fig. 5]).[35] [36] Usually, the imaging modality that is used to assess the primary lesion can also evaluate the regional metastases. Most frequent site of distant metastases is lung and many times they tend to cavitate. Routine chest radiograph can detect overt lung metastases, while for detection of smaller or subpleural lesions, CECT is mandatory.[37] Furthermore, one can miss lesions in the hidden areas of radiograph. FDG-PET-CT has incremental value in detection of subtle metastases and detecting extrathoracic metastases. In addition, it also provides the standardized uptake value.[38]
|
Direct signs |
Indirect signs |
|---|---|
|
1.Nerve enhancement 2.Nerve enlargement 3.Foramina fat plane obliteration 4.Foramina enlargement and destruction 5.Intracranial spread |
1. Denervation atrophy. 2. Denervation enhancement In acute or subacute phase, T2 hyperintense edema in the corresponding muscle develops postcontrast enhancement. In chronic phase, muscle atrophy shows hyperintense signal on T1 and T2-weighted sequences due to fatty replacement |
|
Nerves |
Key findings |
|---|---|
|
1.Ophthalmic branch of trigeminal nerve(V1) |
Obliteration of the orbital fat pad and enhancement of V1 in the orbit and the cavernous sinus |
|
2. Maxillary branch of the trigeminal nerve(V2) |
Obliteration of the fat pads in the pterygopalatine fossa; enlargement of the infraorbital fissure and thickening and enhancement of V2 in the round foramen and cavernous sinus |
|
3. Mandibular branch of the trigeminal nerve(V3) |
Obliteration of the fat pads of the mental or mandibular foramen and of the parapharyngeal fat below the foramen ovale; enlargement or erosion of foramina; thickening and enhancement of V3 in the parapharyngeal space and foramen ovale; abnormal bone marrow in the jaw; signs of denervation of the masticatory muscles |
|
4. Trigeminal nerve |
Obliteration of Meckel's cave |
|
5. Facial nerve |
Obliteration of the fat pad of the stylomastoid foramen and abnormal enhancement |
|
6. Geniculate ganglion |
Enlargement, obliteration and sclerosis of the geniculate fossa |
|
7. Greater superficial petrosal nerve |
Obliteration of the fat pad and enlargement or erosion of the vidian canal |
|
8. Auriculotemporal nerve |
Tumor growth in the posterior mandible |
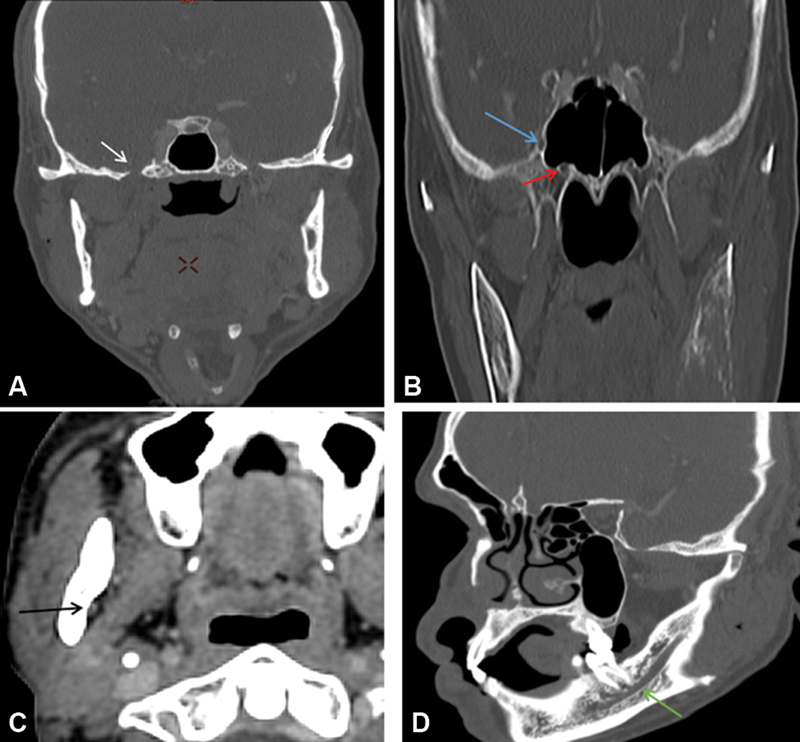
| Figure 3:Multiplanar reformatted images showing normal structures acting as conduit for perineural spread (A–D) foramen ovale (white arrow), foramen rotundum (blue arrow), vidian canal (red arrow), mandibular foramen (black arrow), and inferior alveolar canal (green arrow).

| Figure 4:(A) Axial postcontrast magnetic resonance imaging showing right upper Guillain-Barre syndrome mass with associated enhancement along right mandibular nerve in mandibular foramen suggesting perineural spread (red arrow). (B) Coronal postcontrast image showing enhancement along the infratemporal fossa component of the left mandibular (blue arrow). (C and D) Coronal postcontrast image showing enhancement and thickening of the right mandibular nerve involving foramen and suspicious intracranial extension superiorly (white arrowheads and arrow).
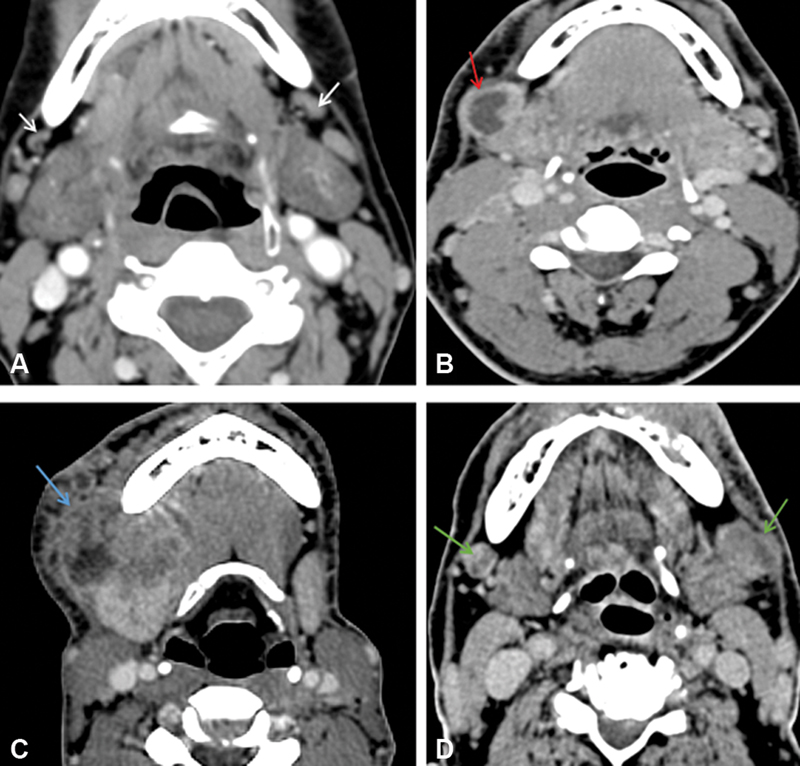
| Figure 5:(A) Bilateral reactive nodes are seen with maintained fatty hilum (white arrow). (B) Necrosis is seen in the right level IB node (red arrow). (C) Right level IB node with extranodal extension is seen (blue arrow). (D) Metastatic bilateral necrotic IB nodes are seen with capsular irregularity in the left IB node suspicious extranodal extension (green arrow).
Management
The various treatment modalities available for oral cavity cancers are surgery, chemotherapy, and radiation therapy either as single modality or in combination. Response assessment in neoadjuvant, adjuvant, and palliative settings aims at the detection of the residual disease and documents increase or decrease in disease burden and presence of new metastases.[17] Response assessment in most of the oral cavity cancers is best done with CECT as it can better detect the presence of residual disease ([Fig. 6]). CE-MRI is the ideal modality for assessing response for oral tongue lesions. Surgical resection or radiation therapy is known to cause various post-treatment changes in the tissue distortion and these changes should be kept in mind while reporting.[39] [40] There are some key findings in gingivobuccal sulcus and tongue cancers on imaging that have vital implications for the management plan. Gingivobuccal sulcus cancers with low ITF involvement on imaging are resectable. High ITF involvement is a relative contraindication for surgery with posterior high ITF (pterygopalatine fossa and pterygomaxillary fissure) involvement requiring palliative care, while anterior high ITF (retroantral fat) involvement is still amenable for surgery. Superficial/cortical bone erosion does not alter the T stage of the disease. Mandible preserving surgery can be done if anteroposterior extent of paramandibular soft tissue is less than 1 cm and directs marginal mandibulectomy if it is more than 1 cm. Deep cortical erosion or marrow involvement upstages the disease to T4a and requires segmental mandibulectomy. Perineural spread of disease is resectable if limited to infra-notch compartment but warrants palliative management if supra-notch extension is present. In tongue cancers, when tumor thickness is more than 4 mm elective neck dissection is done in view of the greater risk of nodal metastases. DOI of more than 10 mm is a marker of poor prognosis for which adjuvant treatment is recommended. When disease crosses the midline contralateral neck, dissection and radiation are warranted. Total glossectomy with flap reconstruction has to be done when bilateral neurovascular bundles get involved. Bone erosion in tongue cancers requires mandibulectomy with reconstruction and invasion of FOM reconstruction with flaps. Involvement of vallecula, pre-epiglottic space, and hyoid are relative contraindications for surgery. Extension to masticator space deems the disease nonresectable. Imaging in a clinically node-negative disease helps to pick up occult/skip nodal metastases that warrants elective neck dissection. High nodal burden on CT warrants PET-CT/CT thorax in view of increased risk of distant metastases. Adjuvant treatment is required if ENE is present.
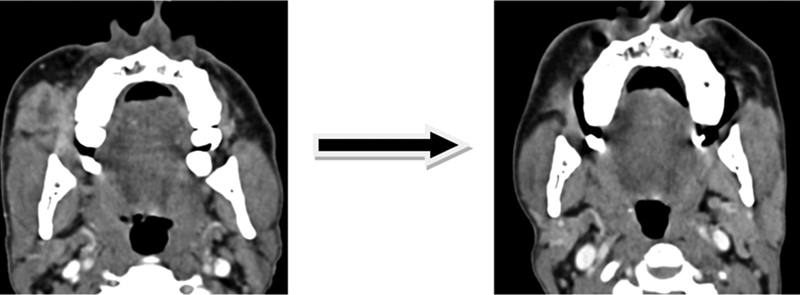
| Figure 16:Axial multidetector computed tomography images showing downstaging of T4b disease post induction chemotherapy.
Follow-Up
Follow-up or surveillance in post-treatment setting aims at the detection of recurrence at the earliest. The timeline for first post-treatment surveillance imaging is usually 3 months post-treatment. However, the National Comprehensive Cancer Network criteria suggest that first surveillance imaging should be performed between 3 and 6 months of completion of therapy.[41] While CECT is preferred for majority of the oral cavity subsites, CE-MRI is preferred for tongue imaging. Radiologists needs to be aware of the possible post-treatment appearance following surgeries or radiation therapy in head and neck as they cause distortion of normal anatomy and fibrosis that make detection of residual or recurrent disease challenging. FDG-PET-CT can be used as a problem-solving tool in distinguishing recurrent tumor from post-treatment changes.[42] [43]
Principles of Management
The mainstay of treatment for oral cancers is surgery with or without adjuvant therapy. For early-stage disease (stages I and II), the treatment is single modality, whereas for advanced stage disease (stages III and IVA), the treatment is multimodality.[17] The primary disease is excised with adequate margin of more than 5 mm all around the tumor. Neck dissection is performed in all cases. Elective neck dissection clearing level I to III is performed for node negative neck. Modified radical neck dissection clearing level I to IV or V is performed preserving all the nonlymphatic structures namely internal jugular vein, spinal accessory nerve, and sternocleidomastoid muscle that are sacrificed only if involved by the disease. For advanced disease (stages III and IV), it is surgical resection followed by radiation therapy with or without concurrent chemotherapy. Inoperable cases are directly treated with radiation therapy with or without concurrent chemotherapy.[44] Best supportive care is recommended if the general condition of the patient is poor precluding any treatment. The stage-wise prognosis (5-year survival rate) in oral cavity cancers is 85.2, 82.9, 56.3, and 42.6%-for stages I, II, III, and IV respectively.[45]
Follow-Up Imaging and Management of Recurrent Disease Including Specific Interventional and Palliative Measures
The main aim of follow-up imaging is diagnosing and treating the recurrent disease at the earliest. Most cases of oral cavity malignancy recur either in the postoperative bed or in the cervical lymph nodes. Image-guided tissue sampling plays an important role in documenting these recurrences. Surgery should be offered to the patients if the recurrence is excisable. However, when the recurrence cannot be excised with clear margins or in the presence of distant metastasis, nonsurgical treatment should be offered that includes chemotherapy or chemoradiation. These recurrent tumors tend to be resistant to many conventional chemotherapeutic drugs and immunotherapy and targeted therapies could be the options for such patients. There is some emerging data that oligometastatic cases with solitary lung recurrence can be treated with radio frequency or microwave ablation, metastasectomy, or stereotactic body radiotherapy; however, the practice varies across the globe.[46] [47]
Summary of Recommendations
1. Oral cavity cancers have better outcomes if detected early and treated with timely surgery.
2. Imaging plays a crucial role in diagnosing, staging the disease, treatment planning on case-to-case basis, and surveillance of disease.
3. Modality of choice for majority of oral cavity cancers is CECT, while for oral tongue and FOM, CE-MRI is performed.
4. FDG-PET-CT is used as a problem-solving tool and in the setting of recurrent or residual disease.
Conflict of Interest
None declared.
The article is not under consideration for publication elsewhere. Each author participated sufficiently for the work to be submitted. Publication is approved by all authors.
Supplementary MaterialReferences
- Starzyńska A, Sobocki BK, Alterio D. Current challenges in head and neck cancer management. Cancers (Basel) 2022; 14 (02) 358
- Goel B, Tiwari AK, Pandey RK. et al. Therapeutic approaches for the treatment of head and neck squamous cell carcinoma-an update on clinical trials. Transl Oncol 2022; 21: 101426
- Kato H, Matsuo M. Imaging findings of oral cancers. In Inflammation and Oral Cancer. Academic Press; 2022: 55-77
- Asthana S, Labani S, Kailash U, Sinha DN, Mehrotra R. Association of smokeless tobacco use and oral cancer: a systematic global review and meta-analysis. Nicotine Tob Res 2019; 21 (09) 1162-1171
- Epstein JB, Barasch A. Oral and dental health in head and neck cancer patients. In Multidisciplinary Care of the Head and Neck Cancer Patient. Springer, Cham; 2018: 43-57
- Haider T, Yousaf Z, Khan AG, Fatima S. Awareness and practice of oral hygiene and its relation to socio-demographic factors among patients attending general OPD: Oral Hygiene & its Relation to Socio-Demographic Factors. Pakistan BioMedical Journal. 2022; 5 (02) 80-83
- Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol 2019; 13 (03) 423-439
- Sarode G, Maniyar N, Sarode SC, Jafer M, Patil S, Awan KH. Epidemiologic aspects of oral cancer. Dis Mon 2020; 66 (12) 100988
- Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade–an update (2000-2012). Asian Pac J Cancer Prev 2013; 14 (10) 5567-5577
- Wong T, Yap T, Wiesenfeld D. Common benign and malignant oral mucosal disease. Aust J Gen Pract 2020; 49 (09) 568-573
- Acharya B, Rayamajhi A. Imaging modalities in squamous cell carcinoma of head and neck: a review article. J Can Res Adv Ther. 2020; 1 (02) 19-23
- Zanoni DK, Patel SG. New AJCC: how does it impact oral cancers?. Oral Oncol 2020; 104: 104607
- Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep 2019; 21 (06) 52
- Grégoire V, Boisbouvier S, Giraud P, Maingon P, Pointreau Y, Vieillevigne L. Management and work-up procedures of patients with head and neck malignancies treated by radiation. Cancer Radiother 2022; 26 (1-2): 147-155
- Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int 2020; 1: 100046
- Yete S, D'Souza W, Saranath D. High-risk human papillomavirus in oral cancer: clinical implications. Oncology 2018; 94 (03) 133-141
- D'Cruz AK, Vaish R, Dhar H. Oral cancers: current status. Oral Oncol 2018; 87: 64-69
- Mahajan A, Ahuja A, Sable N, Stambuk HE. Imaging in oral cancers: a comprehensive review. Oral Oncol 2020; 104: 104658
- Jeyaraj PR, Samuel Nadar ER. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J Cancer Res Clin Oncol 2019; 145 (04) 829-837
- Ilhan B, Lin K, Guneri P, Wilder-Smith P. Improving oral cancer outcomes with imaging and artificial intelligence. J Dent Res 2020; 99 (03) 241-248
- Junn JC, Soderlund KA, Glastonbury CM. Imaging of head and neck cancer with CT, MRI, and US. In Seminars in Nuclear Medicine. WB Saunders: 2021. Jan 1 (Vol. 51, No. 1, pp. 3–12)
- Murakami R, Shiraishi S, Yoshida R. et al. Reliability of MRI-derived depth of invasion of oral tongue cancer. Acad Radiol 2019; 26 (07) e180-e186
- Maraghelli D, Pietragalla M, Calistri L. et al. Techniques, tricks, and stratagems of oral cavity computed tomography and magnetic resonance imaging. Appl Sci (Basel) 2022; 12 (03) 1473
- Eneva MI, Nedevska M. MDCT amplified by the “puffed-cheek” maneuver in the radiological assessment of oral cavity tumors–a technical guide and a pictorial review of imaging findings. Vienna: European Congress of Radiology-ECR; 2018
- Lee YC, Jung AR, Kwon OE. et al. Comparison of computed tomography, magnetic resonance imaging, and positron emission tomography and computed tomography for the evaluation bone invasion in upper and lower gingival cancers. J Oral Maxillofac Surg 2019; 77 (04) 875.e1-875.e9
- Mahajan A, Dhone N, Vaish R. et al. Prognostic impact of pattern of mandibular involvement in gingivo-buccal complex squamous cell carcinomas: marrow and mandibular canal staging system. Front Oncol 2022; 11: 752018
- Mao MH, Wang S, Feng ZE. et al. Accuracy of magnetic resonance imaging in evaluating the depth of invasion of tongue cancer. A prospective cohort study. Oral Oncol 2019; 91: 79-84
- Aiken AH. Image-guided biopsies in the head and neck: practical value and approach. AJNR Am J Neuroradiol 2020; 41 (11) 2123-2125
- Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol 2015; 59 (05) 578-585
- Hanna E, Vural E, Prokopakis E, Carrau R, Snyderman C, Weissman J. The sensitivity and specificity of high-resolution imaging in evaluating perineural spread of adenoid cystic carcinoma to the skull base. Arch Otolaryngol Head Neck Surg 2007; 133 (06) 541-545
- Mahajan A, Smriti VM. Masseter pars coronoidea: Gateway between the masticator space and infratemporal fossa. Cancer Res Stat Treat 2022; 5 (01) 185
- Mohiyuddin SMA, Harsha P, Maruvala S. et al. Outcome of compartment resection of locally advanced oral cancers extending to infratemporal fossa: a tertiary rural hospital experience. Eur Arch Otorhinolaryngol 2018; 275 (11) 2843-2850
- Arya S, Chaukar D, Pai P. Imaging in oral cancers. Indian J Radiol Imaging 2012; 22 (03) 195-208
- Liao CT, Ng SH, Chang JT. et al. T4b oral cavity cancer below the mandibular notch is resectable with a favorable outcome. Oral Oncol 2007; 43 (06) 570-579
- Pantvaidya G, Rao K, D'Cruz A. Management of the neck in oral cancers. Oral Oncol 2020; 100: 104476
- Abdel-Halim CN, Rosenberg T, Dyrvig AK. et al. Diagnostic accuracy of imaging modalities in detection of histopathological extranodal extension: A systematic review and meta-analysis. Oral Oncol 2021; 114: 105169
- Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent 2016; 6 (04) 265-271
- Ishikawa S, Hiraka T, Kirii K. et al. Relationship between standard uptake values of positron emission tomography/computed tomography and salivary metabolites in oral cancer: a pilot study. J Clin Med 2020; 9 (12) 3958
- Dhar H, Vaish R, D'Cruz AK. Management of locally advanced oral cancers. Oral Oncol 2020; 105: 104662
- Geiger JL, Adelstein DJ. Chemotherapy in the definitive management of oral cancers: where do we stand today?. Oral Oncol 2020; 102: 104584
- Strauss SB, Aiken AH, Lantos JE, Phillips CD. Best practices: application of NI-RADS for posttreatment surveillance imaging of head and neck cancer. AJR Am J Roentgenol 2021; 216 (06) 1438-1451
- Dinkelborg P, Ro SR, Shnayien S. et al. Retrospective evaluation of NI-RADS for detecting postsurgical recurrence of oral squamous cell carcinoma on surveillance CT or MRI. AJR Am J Roentgenol 2021; 217 (01) 198-206
- Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. Post-treatment 18F-FDG PET/CT for predicting survival and recurrence in patients with advanced-stage head and neck cancer undergoing curative surgery. Oral Oncol 2020; 107: 104750
- Koyfman SA, Ismaila N, Crook D. et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol 2019; 37 (20) 1753-1774
- Bobdey S, Sathwara J, Jain A, Saoba S, Balasubramaniam G. Squamous cell carcinoma of buccal mucosa: an analysis of prognostic factors. South Asian J Cancer 2018; 7 (01) 49-54
- Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: raising the bar a step higher. Oral Oncol 2020; 101: 104492
- Swain M, Ghosh-Laskar S. Stereotactic body radiotherapy (SBRT) for primary non-metastatic head and neck cancer: when less is enough. Oral Oncol 2021; 116: 105265

| Figure 1:(A) Axial section shows an ill-defined thickening involving right buccal mucosa buccinator complex with loss of fat planes with masseter muscle (white arrow). (B) Sagittal oblique shows an ill-defined thickening involving retromolar trigone region (green arrow) highlighting importance of oblique reformation. (C) Coronal reformatted images show technique to measure

| Figure 2:Oblique sagittal reformatted images showing superficial cortical erosion (white arrow) of the mandible, (B) erosion of the coronoid process of the mandible (Green arrow), (C) cortical and medullary erosion with involvement of inferior alveolar canal (black arrow) of mandible. (D) Shaded surface display image shows erosion of mandible and important in planning surgical reconstruction.

| Figure 3:Multiplanar reformatted images showing normal structures acting as conduit for perineural spread (A–D) foramen ovale (white arrow), foramen rotundum (blue arrow), vidian canal (red arrow), mandibular foramen (black arrow), and inferior alveolar canal (green arrow).

| Figure 4:(A) Axial postcontrast magnetic resonance imaging showing right upper Guillain-Barre syndrome mass with associated enhancement along right mandibular nerve in mandibular foramen suggesting perineural spread (red arrow). (B) Coronal postcontrast image showing enhancement along the infratemporal fossa component of the left mandibular (blue arrow). (C and D) Coronal postcontrast image showing enhancement and thickening of the right mandibular nerve involving foramen and suspicious intracranial extension superiorly (white arrowheads and arrow).

| Figure 5:(A) Bilateral reactive nodes are seen with maintained fatty hilum (white arrow). (B) Necrosis is seen in the right level IB node (red arrow). (C) Right level IB node with extranodal extension is seen (blue arrow). (D) Metastatic bilateral necrotic IB nodes are seen with capsular irregularity in the left IB node suspicious extranodal extension (green arrow).

| Figure 16:Axial multidetector computed tomography images showing downstaging of T4b disease post induction chemotherapy.
References
- Starzyńska A, Sobocki BK, Alterio D. Current challenges in head and neck cancer management. Cancers (Basel) 2022; 14 (02) 358
- Goel B, Tiwari AK, Pandey RK. et al. Therapeutic approaches for the treatment of head and neck squamous cell carcinoma-an update on clinical trials. Transl Oncol 2022; 21: 101426
- Kato H, Matsuo M. Imaging findings of oral cancers. In Inflammation and Oral Cancer. Academic Press; 2022: 55-77
- Asthana S, Labani S, Kailash U, Sinha DN, Mehrotra R. Association of smokeless tobacco use and oral cancer: a systematic global review and meta-analysis. Nicotine Tob Res 2019; 21 (09) 1162-1171
- Epstein JB, Barasch A. Oral and dental health in head and neck cancer patients. In Multidisciplinary Care of the Head and Neck Cancer Patient. Springer, Cham; 2018: 43-57
- Haider T, Yousaf Z, Khan AG, Fatima S. Awareness and practice of oral hygiene and its relation to socio-demographic factors among patients attending general OPD: Oral Hygiene & its Relation to Socio-Demographic Factors. Pakistan BioMedical Journal. 2022; 5 (02) 80-83
- Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol 2019; 13 (03) 423-439
- Sarode G, Maniyar N, Sarode SC, Jafer M, Patil S, Awan KH. Epidemiologic aspects of oral cancer. Dis Mon 2020; 66 (12) 100988
- Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade–an update (2000-2012). Asian Pac J Cancer Prev 2013; 14 (10) 5567-5577
- Wong T, Yap T, Wiesenfeld D. Common benign and malignant oral mucosal disease. Aust J Gen Pract 2020; 49 (09) 568-573
- Acharya B, Rayamajhi A. Imaging modalities in squamous cell carcinoma of head and neck: a review article. J Can Res Adv Ther. 2020; 1 (02) 19-23
- Zanoni DK, Patel SG. New AJCC: how does it impact oral cancers?. Oral Oncol 2020; 104: 104607
- Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep 2019; 21 (06) 52
- Grégoire V, Boisbouvier S, Giraud P, Maingon P, Pointreau Y, Vieillevigne L. Management and work-up procedures of patients with head and neck malignancies treated by radiation. Cancer Radiother 2022; 26 (1-2): 147-155
- Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int 2020; 1: 100046
- Yete S, D'Souza W, Saranath D. High-risk human papillomavirus in oral cancer: clinical implications. Oncology 2018; 94 (03) 133-141
- D'Cruz AK, Vaish R, Dhar H. Oral cancers: current status. Oral Oncol 2018; 87: 64-69
- Mahajan A, Ahuja A, Sable N, Stambuk HE. Imaging in oral cancers: a comprehensive review. Oral Oncol 2020; 104: 104658
- Jeyaraj PR, Samuel Nadar ER. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J Cancer Res Clin Oncol 2019; 145 (04) 829-837
- Ilhan B, Lin K, Guneri P, Wilder-Smith P. Improving oral cancer outcomes with imaging and artificial intelligence. J Dent Res 2020; 99 (03) 241-248
- Junn JC, Soderlund KA, Glastonbury CM. Imaging of head and neck cancer with CT, MRI, and US. In Seminars in Nuclear Medicine. WB Saunders: 2021. Jan 1 (Vol. 51, No. 1, pp. 3–12)
- Murakami R, Shiraishi S, Yoshida R. et al. Reliability of MRI-derived depth of invasion of oral tongue cancer. Acad Radiol 2019; 26 (07) e180-e186
- Maraghelli D, Pietragalla M, Calistri L. et al. Techniques, tricks, and stratagems of oral cavity computed tomography and magnetic resonance imaging. Appl Sci (Basel) 2022; 12 (03) 1473
- Eneva MI, Nedevska M. MDCT amplified by the “puffed-cheek” maneuver in the radiological assessment of oral cavity tumors–a technical guide and a pictorial review of imaging findings. Vienna: European Congress of Radiology-ECR; 2018
- Lee YC, Jung AR, Kwon OE. et al. Comparison of computed tomography, magnetic resonance imaging, and positron emission tomography and computed tomography for the evaluation bone invasion in upper and lower gingival cancers. J Oral Maxillofac Surg 2019; 77 (04) 875.e1-875.e9
- Mahajan A, Dhone N, Vaish R. et al. Prognostic impact of pattern of mandibular involvement in gingivo-buccal complex squamous cell carcinomas: marrow and mandibular canal staging system. Front Oncol 2022; 11: 752018
- Mao MH, Wang S, Feng ZE. et al. Accuracy of magnetic resonance imaging in evaluating the depth of invasion of tongue cancer. A prospective cohort study. Oral Oncol 2019; 91: 79-84
- Aiken AH. Image-guided biopsies in the head and neck: practical value and approach. AJNR Am J Neuroradiol 2020; 41 (11) 2123-2125
- Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol 2015; 59 (05) 578-585
- Hanna E, Vural E, Prokopakis E, Carrau R, Snyderman C, Weissman J. The sensitivity and specificity of high-resolution imaging in evaluating perineural spread of adenoid cystic carcinoma to the skull base. Arch Otolaryngol Head Neck Surg 2007; 133 (06) 541-545
- Mahajan A, Smriti VM. Masseter pars coronoidea: Gateway between the masticator space and infratemporal fossa. Cancer Res Stat Treat 2022; 5 (01) 185
- Mohiyuddin SMA, Harsha P, Maruvala S. et al. Outcome of compartment resection of locally advanced oral cancers extending to infratemporal fossa: a tertiary rural hospital experience. Eur Arch Otorhinolaryngol 2018; 275 (11) 2843-2850
- Arya S, Chaukar D, Pai P. Imaging in oral cancers. Indian J Radiol Imaging 2012; 22 (03) 195-208
- Liao CT, Ng SH, Chang JT. et al. T4b oral cavity cancer below the mandibular notch is resectable with a favorable outcome. Oral Oncol 2007; 43 (06) 570-579
- Pantvaidya G, Rao K, D'Cruz A. Management of the neck in oral cancers. Oral Oncol 2020; 100: 104476
- Abdel-Halim CN, Rosenberg T, Dyrvig AK. et al. Diagnostic accuracy of imaging modalities in detection of histopathological extranodal extension: A systematic review and meta-analysis. Oral Oncol 2021; 114: 105169
- Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent 2016; 6 (04) 265-271
- Ishikawa S, Hiraka T, Kirii K. et al. Relationship between standard uptake values of positron emission tomography/computed tomography and salivary metabolites in oral cancer: a pilot study. J Clin Med 2020; 9 (12) 3958
- Dhar H, Vaish R, D'Cruz AK. Management of locally advanced oral cancers. Oral Oncol 2020; 105: 104662
- Geiger JL, Adelstein DJ. Chemotherapy in the definitive management of oral cancers: where do we stand today?. Oral Oncol 2020; 102: 104584
- Strauss SB, Aiken AH, Lantos JE, Phillips CD. Best practices: application of NI-RADS for posttreatment surveillance imaging of head and neck cancer. AJR Am J Roentgenol 2021; 216 (06) 1438-1451
- Dinkelborg P, Ro SR, Shnayien S. et al. Retrospective evaluation of NI-RADS for detecting postsurgical recurrence of oral squamous cell carcinoma on surveillance CT or MRI. AJR Am J Roentgenol 2021; 217 (01) 198-206
- Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. Post-treatment 18F-FDG PET/CT for predicting survival and recurrence in patients with advanced-stage head and neck cancer undergoing curative surgery. Oral Oncol 2020; 107: 104750
- Koyfman SA, Ismaila N, Crook D. et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol 2019; 37 (20) 1753-1774
- Bobdey S, Sathwara J, Jain A, Saoba S, Balasubramaniam G. Squamous cell carcinoma of buccal mucosa: an analysis of prognostic factors. South Asian J Cancer 2018; 7 (01) 49-54
- Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: raising the bar a step higher. Oral Oncol 2020; 101: 104492
- Swain M, Ghosh-Laskar S. Stereotactic body radiotherapy (SBRT) for primary non-metastatic head and neck cancer: when less is enough. Oral Oncol 2021; 116: 105265


 PDF
PDF  Views
Views  Share
Share

