Immune-Related Adverse Events (irAEs) in Cancer, with Inputs from a Nursing Expert: A Review
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(02): 144-152
DOI: DOI: 10.1055/s-0042-1742442
Abstract
Immune checkpoint inhibitors (ICPis) belong to a group of immunotherapeutic agents that act on different immune cells and tumor cells and reactivate the suppressed immune system of the host. The emergence of immunotherapy has resulted in the successful management of many malignancies. High success rates with certain advanced cancers have attributed wide importance and relevance to the use of immunotherapy. Although ICPis have gained huge popularity, their use often leads to side effects that can affect almost any system; immune-related adverse events (irAEs). These adverse events occur due to unrestrained T cell activity that unsettles the immune homeostasis of the host. Although close monitoring for toxicities controls the events on most of the occasions, the inability to diagnose them early may prove fatal on some occasions due to their subtle and nonspecific symptoms. This review summarizes in brief the usual irAEs and their management, besides a very important nursing perspective, from a nursing expert about an overall insight into the routine irAEs.
Keywords
immune-related adverse events - immune checkpoint inhibitors - immunosuppressant - cytotoxic T lymphocyte antigen 4 - programmed cell death protein 1Source(s) of Support
None.
Presentation at a Meeting
None.
Publication History
Article published online:
13 April 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Immune checkpoint inhibitors (ICPis) belong to a group of immunotherapeutic agents that act on different immune cells and tumor cells and reactivate the suppressed immune system of the host. The emergence of immunotherapy has resulted in the successful management of many malignancies. High success rates with certain advanced cancers have attributed wide importance and relevance to the use of immunotherapy. Although ICPis have gained huge popularity, their use often leads to side effects that can affect almost any system; immune-related adverse events (irAEs). These adverse events occur due to unrestrained T cell activity that unsettles the immune homeostasis of the host. Although close monitoring for toxicities controls the events on most of the occasions, the inability to diagnose them early may prove fatal on some occasions due to their subtle and nonspecific symptoms. This review summarizes in brief the usual irAEs and their management, besides a very important nursing perspective, from a nursing expert about an overall insight into the routine irAEs.
Keywords
Introduction
Advances in the treatment of cancer have led to the development of immune checkpoint inhibitors (ICPis) that reinforce the immune system to target tumor cells. The agents, cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1) inhibitors, have contributed for the effective treatment of advanced cancers in the recent times.[1] The beneficial clinical responses of such agents against various malignancies have resulted further in U.S. Food and Drug Administration approval of checkpoint inhibitors, pembrolizumab,[2] nivolumab,[3] cemiplimab,[4] atezolizumab,[5] durvalumab,[6] and avelumab.[7] These agents are approved for several malignancies including melanoma, renal cell carcinoma, lung cancer (small and nonsmall cell types), bladder cancer, and Hodgkin disease. Currently several clinical trials are further investigating the combination therapies involving checkpoint inhibitors, targeted therapy, radiotherapy, chemotherapy, and various antiangiogenic agents, with few of these combinations already approved and used in clinical practice.[8] ICPis are generally well tolerated and less toxic than the cytotoxic chemotherapy. These agents, however, function by recalibrating the immune cell functions by blocking the internal down-regulators of the immune system, resulting in a distinctive group of side effects referred to as immune-related adverse events (irAEs). These are different from the side effects seen with conventional cancer therapy.[9] [10] The adverse events can be seen in most of the organs due to the heightened immune system, and thus organs like liver, skin, bowels, kidneys, endocrine tissues, and the central nervous system are affected ([Fig. 1]).[11] The irAEs are diverse and range from a mild dermatitis to fatal pneumonitis and myocarditis. Higher rates of irAEs are seen with ICPis targeting CTLA-4 than those acting on PD-1.[12] [13] Most irAEs develop during the initial 3 to 4 months of immunotherapy use, although the events can manifest any time during the course of treatment or sometimes even after the conclusion of immunotherapy treatment. The initial events often include nonspecific symptoms like fatigue and malaise. Patients often presume them to be anticipated with the disease process, and if not documented could result in delayed diagnosis. Therefore, careful observation of patients under ICPis becomes important for the differential diagnosis, and effective management of any events.
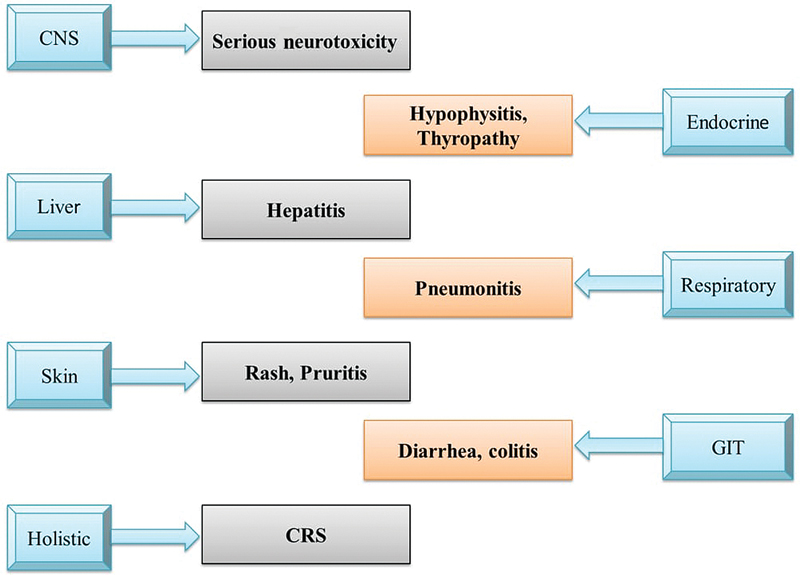
| Fig. 1 Involvement of various systems by immune- related adverse events. CNS, central nervous system; CRS, cytokine release syndrome; GIT, gastrointestinal tract.
CTLA-4 and PD-1/PD-L1 negatively regulate and maintain the balance in the immune system under normal conditions. T cell surfaces express CTLA-4 and CD28 that compete on the surface of antigen presenting cells (APCs) with the same binding sites as CD80/CD86. The activation signal for T cells is achieved when CD28 integrates with CD80/CD86.[14] The activation of T cells is obstructed with down-regulation of their responses when CTLA-4 combines with CD80/CD86.[15] [16] PD-1 and its ligand PD-L1 are expressed on the surface T cells, and the surface of APCs, respectively. The down-regulation of the T cell response also occurs when PD-1 and PD-L1 integrate on the tumor cell surface thereby enabling tumor cells to evade immune response of the host.[17] [18] These immune inhibitor molecules are up-regulated by the tumor cells to escape the immune response. This ultimately facilitates the initiation of the tumor, followed by the stages of progression and metastasis. The uses of ICPis in cancer treatment block PD1/PD-L1 or CTLA-4 and thus stimulate the body's antitumor immunity disrupting immune homeostasis and also make patients susceptible for irAEs. The class of ICPis used dictates the incidence and extent of these irAEs. The use of ipilimumab that inhibits CTLA-4 caused irAEs in up to 60 to 70% of patients,[1] while the incidence of irAEs is seen in 30% of patients under PD-1 inhibitors. The combination of ipilimumab and another PD-1 inhibitor nivolumab has shown the highest incidence of irAEs.[19]
Various IrAEs and Their Management
Skin
The most common ICPis induce dermatological reactions that include rash, vitiligo, and pruritis. The dermatologic toxicities are the earliest irAEs encountered, appearing at an average of 3.6 weeks after ICPis initiation.[20] It is recommended to undertake a thorough clinical examination of the mucocutaneous surfaces to determine the type and spread of the lesions,[21] for the purpose of differential diagnosis.[22] Topical emollients and topical steroids are the mainstay for the mild rashes secondary to the use of ICPis. Cold packs and oatmeal baths have been successfully used for relieving pruritis.[23] Moderate cases affecting quality of life are treated with oral antihistamines along with medium to high potency topical corticosteroids; the treatment continues until lesions return to grade 1.[22] If the lesions continue unabated despite the interventions, ICPis are withheld and patients are referred for dermatology consultations to determine recovery prospects from irAEs.[23]
Respiratory
ICPis therapy can lead to pneumonitis (interstitial lung disease) on rare occasions.[24] The various clinical trials have reported a pneumonitis incidence of 1%-due to ipilimumab, 3 to 5%-with anti PD-1 and anti-PD-L1 monotherapy.[25] The incidence soars up to 10%-in combination therapy with anti PD-1 or anti-PD-L1 and CTLA-4 inhibitors.[26] [27] [28] [29] [30] The main investigations required for the diagnosis of immune-mediated pneumonitis include pulse oximetry, chest X-ray, and computed tomography (CT). Radiographic evidence is used to evaluate grade 1 pneumonitis and the disease progression is assessed by a CT at 3 to 4 weeks. In moderate-to-severe cases, ICPis are terminated till recovery or until reversal to grade 1 toxicity, with administration of prednisone in grade 2 pneumonitis.[31] In addition to aborting of ICPis, it is recommended to administer appropriate antibiotics and prednisolone if the toxicity is progressing to either grade 3 or to grade 4.[22] [32] ICPis-related pneumonitis has four patterns, organizing pneumonia, nonspecific interstitial pneumonia, hypersensitivity pneumonitis, and diffuse alveolar damage ([Table 1]).[25]
|
Type |
Clinical features |
Radiology |
Histopathology |
Management |
|---|---|---|---|---|
|
OP |
Nonproductive cough Shortness of breath Loss of weight (< 2> |
Peripheral areas of ground glass opacities, multiple, solitary, infiltrative alveolar opacities |
Distal bronchi and alveoli involvement with granulation tissue Plasma cells and lymphocytes |
Mild Spontaneous recovery may occur Close monitoring of pulmonary functions required Progressive/and or persistent Prednisone 0.5–1.0mg/kg/day (3–6 months) |
|
NSIP |
Nonproductive cough, dyspnea, developing over weeks to months. Bibasilar crackles |
Reticular markings, traction bronchiectasis, and ground-glass opacities are seen mostly in lower zones |
Fibrosis with diffuse infiltrative cell infiltrate, alveolar walls are uniformly and diffusely thickened, alveolar structural integrity is maintained |
Mild Pulmonary function observation Moderate 0.5–1.0mg/kg/day prednisone or equivalent (8–12 weeks) Refractory disease IV corticosteroids and/or cytotoxic therapies |
|
DAD |
Quick onset severe dyspnea, cough |
Extensive air space opacities, more in the dependent areas |
Alveolar thickening with inflammatory infiltrate |
Respiratory failure Supportive therapies High-dose IV corticosteroids |
|
Routine procedural pretreatment |
History ▪ Gather information about infectious, autoimmune, endocrine, and any organ specific disease history ▪ Enquire about baseline bowel habit (frequency and stool consistency) Blood tests ▪ CBC ▪ CMP ▪ TSH ▪ FreeT4 ▪ HbA1c ▪ Total CK ▪ Fasting lipid profile ▪ HBsAg, HBcAb, HBsAb, CMV antibody, hCAb, T-spot test, HIV antigen(p24)[a] Dermatological examination ▪ Mucocutaneous examination, with careful observation of the extent and type of the lesion Pulmonary test ▪ Baseline oxygen saturation on room air and during ambulation Cardiac tests ▪ ECG ▪ Troponin I or T: baseline and weekly for 6 weeks[b] |
|
Additional screening tests in pre-existing organ disease/at risk of organ-specific toxicity |
Endocrine tests ▪ Cortisol (8 am) ▪ ACTH (8 am) Cardiac ▪ BNP or NT pro-BNP Pulmonary ▪ PFTs[c] ▪ 6MWT[c] |
Excerpts from a Nursing Expert
Marianne Davies, NP, DNP, MSN, BSN, a lecturer in nursing at the Yale School of Nursing shared her expertise with OncLive, about various aspects of irAEs.[73] According to Marianne Davies, inappropriate management of irAEs can end up in severe colitis and myocarditis. She stressed upon baseline assessment in understanding actual etiology, and stressed upon further laboratory investigations and stool cultures. Prompt steroids and immunosuppressive agents are vital to manage and keep in check the severe irAEs. Davies recommends use of supportive treatment like loperamide or diphenoxylate atropine for the most common low-grade GI toxicity based on the Common Terminology Criteria for Adverse Events. Steroids should be started for grade 2 irAEs toxicity that is associated with increased frequency of stools/day, which is in conformity with their previous guidelines for the management of irAEs. Davies further reported that while updating their guidelines, it is recommended now to use infliximab or vedolizumab if colitis does not show any improvement after 2 weeks of steroids use. The use of these additional immunosuppressive agents is to assist in steroid tapering and hence to safeguard against complications associated with continuous steroid use.
There are higher chances of cardiotoxicity if more than one ICPis are used, such as the combination of an anti-PD-1 and anti-CTLA-4 agent. The patients with underlying cardiovascular disease are more likely to develop such toxicity. Such patients should be monitored closely for any sign indicating toxicity. The patients should also be undertaken for baseline electrocardiograph and troponin levels, such that baseline levels are familiar in case of any changes in evaluation of potential irAEs. If the patients do not respond to the initial steroid therapy, additional immunosuppressant agents should be started within 2 to 3 days to reduce chances of fatal reactions in such susceptible patients.
Davies further highlights the importance of distinguishing irAEs from other nonrelated signs particularly in patients under concurrent treatment. ICPis and chemotherapy are given simultaneously in certain cases, and such combination can lead to overlapping of toxicities. Since the agents are administered together, it is vital to understand the onset and pattern of the symptoms to differentiate the cause and also the approach to the treatment of such toxicities. Chemotherapy-associated peripheral neuropathy may occur at the same time when irAEs occur. The hepatic and renal dysfunction secondary to irAEs may also occur as a result of chemotherapy. The chemotherapy-associated side effects mostly occur at 7 to 14 days of chemotherapy and further take around 14 days to improve, in line with the next chemotherapy treatment time. The irAEs are generally persistent for weeks, take longer time to improve and more so if corticosteroid treatment is delayed. If careful assessment indicates a chemotherapy-related toxicity, the dose of the corresponding agent can be either reduced or withheld for some time depending upon the severity of the associated toxicities. On the other hand, Davies pointed out that irAEs do not warrant a dose reduction, but can be aborted in certain situations and a need for immediate corticosteroid therapy is discussed.
Furthermore, it is important to assess the pattern and mode of onset of toxicities for their appropriate management. In addition to the concurrent therapy, other factors that contribute to the adverse effects may include over-the-counter medications and simultaneous viral infections. Davies stressed upon a thorough review of systems and adequate physical examination for the differential diagnosis, and to efficiently devise the management approaches.
Limitations
The review does not describe the detailed pathogenesis underlying the advent of irAEs.
The management of irAEs is given briefly to accommodate most of the systems description.
Strengths
Although brief, attempts were taken to highlight most of the irAEs and a layout of their management given.
An important section is addressed to the nursing fraternity to raise awareness about the basic aspects of irAEs, and how to begin with treatment as part of a multidisciplinary team.
A set of strategies have been suggested due to a compelling need to develop protocols to manage irAEs effectively ([Table 3]).[74]
|
Action items |
Recommended action plan |
|---|---|
|
Patient confidence building measures |
The information and monitoring of symptoms regarding irAEs should be provided through unique drug-based wallet cards, educational applications, social networking, and through support groups The information should be based on patient preferences, their literacy, psychological, and cultural needs |
|
Refining management guidelines for irAEs |
Organizing an irAEs summit Formation of committees that work on specific toxicities to formulate evidence-based guidelines common to all Involve a multidisciplinary team comprising of emergency doctors, surgeons, anesthesiologists, primary health care doctors, patient advocates, and nurses for the development of guidelines Make public the outcome of the proposed summit Conduct such summits regularly |
|
Systematize irAEs reporting |
SITC CTCAE Task Force Module (irAEs-specific) should be included in any future CTCAE designs |
|
Improving ad standardizing immunosuppressive agents |
Research to be undertaken to evaluate the safety profile and efficacy of immunosuppressants in irAEs treatment. This should be further done to check their impact to ICPis to formulate the choice, dosage, and duration of the use of immunosuppressants to manage irAEs |
|
Steps undertaken to understand underlying mechanisms |
To conduct more studies to understand better the underlying mechanisms for the development of irAEs, determine their possible relationship with management results, recognize factors that predict toxicity, find infection risks and association between ICPis use and emergence of infection, and assess the role of prophylactic vaccines and antibiotic therapy |
|
Conduct studies on vulnerable populations |
Prospective studies should be conducted to assess the safety and efficacy of ICPis in populations with history of immunosuppression[*] or prior irAEs Translation studies for identifying immune markers that forecast response and risk for the development of irAEs The patients and the caregivers should be briefed about all the possibilities before ICPis therapy Selection of immunosuppressants should be optimized to achieve adequate immunosuppression without affecting the benefits of ICPis Specific guidelines for immune check point inhibitor use should be developed in high-risk patients Formulate a national registry of high-risk cancer patients treated with ICPis |
|
Improving diagnostics for efficient irAEs management |
Explore markers to predict likelihood of irAEs Novel tools for patient surveillance Conduct large prospective studies to validate reliable markers that can be applied to general populations |
|
Availing effective means of communication |
Healthcare workers should be trained and equipped to use wireless and digital technologies Promote smartphone-based applications to monitor patients for signs that signal potential irAEs Ability to use immediate protocols based on assembled data |
|
To come up with a platform to record missing patient's voice |
Use of validated tools, e.g., the MD Anderson Symptom Inventory, for analysis of symptoms over a long period of time in different studies, for early detection of irAEs |
|
Propagation of new findings |
Publicize the results of the studies to the scientific community in a timely manner |

| Fig. 1 Involvement of various systems by immune- related adverse events. CNS, central nervous system; CRS, cytokine release syndrome; GIT, gastrointestinal tract.
References
- Martins F, Sofiya L, Sykiotis GP. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019; 16 (09) 563-580
- Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev 2018; 62: 39-49
- Gomes F, Serra-Bellver P, Lorigan P. The role of nivolumab in melanoma. Future Oncol 2018; 14 (13) 1241-1252
- Migden MR, Rischin D, Schmults CD. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379 (04) 341-351
- Schmid P, Adams S, Rugo HS. et al; IMpassion130 Trial Investigators. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379 (22) 2108-2121
- Paz-Ares L, Dvorkin M, Chen Y. et al; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394 (10212): 1929-1939
- Motzer RJ, Penkov K, Haanen J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380 (12) 1103-1115
- Seliger B. Combinatorial approaches with checkpoint inhibitors to enhance anti-tumor immunity. Front Immunol 2019; 10: 999
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33 (17) 1974-1982
- Chen TW, Razak AR, Bedard PL, Siu LL, Hansen AR. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol 2015; 26 (09) 1824-1829
- Liu YH, Zang XY, Wang JC, Huang SS, Xu J, Zhang P. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed Pharmacother 2019; 120: 109437
- Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs 2016; 30 (06) 571-584
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018; 8: 86
- Hou W, Zhou X, Yi C, Zhu H. Immune check point inhibitors and immune-related adverse events in small cell lung cancer. Front Oncol 2021; 11: 604227
- Quandt D, Hoff H, Rudolph M, Fillatreau S, Brunner-Weinzierl MC. A new role of CTLA-4 on B cells in thymus-dependent immune responses in vivo. J Immunol 2007; 179 (11) 7316-7324
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331 (6024): 1565-1570
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer–response. Clin Cancer Res 2013; 19 (19) 5542
- Zhou X, Hou W, Gao L, Shui L, Yi C, Zhu H. Synergies of antiangiogenic therapy and immune checkpoint blockade in renal cell carcinoma: from theoretical background to clinical reality. Front Oncol 2020; 10: 1321
- Wolchok JD, Chiarion-Sileni V, Gonzalez R. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377 (14) 1345-1356 [published correction appears in N Engl J Med. 2018 Nov 29;379(22):2185]
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30 (21) 2691-2697
- Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy front. Front Pharmacol 2017; 8: 49
- Brahmer JR, Lacchetti C, Schneider BJ. et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36 (17) 1714-1768
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016; 2 (10) 1346-1353
- Antoniou KM, Margaritopoulos GA, Tomassetti S, Bonella F, Costabel U, Poletti V. Interstitial lung disease. Eur Respir Rev 2014; 23 (131) 40-54
- Zhong L, Altan M, Shannon VR, Sheshadri A. Immune-related adverse events: pneumonitis. Adv Exp Med Biol 2020; 1244: 255-269
- Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016; 2 (12) 1607-1616
- Khunger M, Rakshit S, Pasupuleti V. et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung Cancer: a systematic review and meta-analysis of trials. Chest 2017; 152 (02) 271-281
- Naidoo J, Wang X, Woo KM. et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35 (07) 709-717
- Robert C, Long GV, Brady B. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372 (04) 320-330
- Reck M, Rodríguez-Abreu D, Robinson AG. et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016; 375 (19) 1823-1833
- Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis 2016; 10 (03) 183-193
- Zhang S, Liang F, Zhu J, Chen Q. Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: a meta-analysis. Mol Cancer Ther 2017; 16 (08) 1588-1595
- Assarzadegan N, Montgomery E, Anders RA. Immune checkpoint inhibitor colitis: the flip side of the wonder drugs. Virchows Arch 2018; 472 (01) 125-133
- Rajha E, Chaftari P, Kamal M, Maamari J, Chaftari C, Yeung SJ. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol Rep (Oxf) 2019; 8 (01) 25-30
- Marin-Acevedo JA, Harris DM, Burton MC. Immunotherapy-induced colitis: an emerging problem for the hospitalist. J Hosp Med 2018; 13 (06) 413-418
- Marthey L, Mateus C, Mussini C. et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohn's Colitis 2016; 10 (04) 395-401
- Haanen JBAG, Carbonnel F, Robert C. et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (Suppl. 04) iv119-iv142
- Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373 (01) 23-34
- Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016; 45: 7-18
- Belli C, Zuin M, Mazzarella L. et al. Liver toxicity in the era of immune checkpoint inhibitors: a practical approach. Crit Rev Oncol Hematol 2018; 132: 125-129
- Hodi FS, O'Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363 (08) 711-723
- Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373 (17) 1627-1639
- Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373 (02) 123-135
- Sanjeevaiah A, Kerr T, Beg MS. Approach and management of checkpoint inhibitor-related immune hepatitis. J Gastrointest Oncol 2018; 9 (01) 220-224
- Anderson B, Morganstein DL. Endocrine toxicity of cancer immunotherapy: clinical challenges. Endocr Connect 2021; 10 (03) R116-R124
- Bai X, Lin X, Zheng K. et al. Mapping endocrine toxicity spectrum of immune checkpoint inhibitors: a disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine 2020; 69 (03) 670-681
- Albarel F, Gaudy C, Castinetti F. et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol 2015; 172 (02) 195-204
- Min L, Hodi FS, Giobbie-Hurder A. et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res 2015; 21 (04) 749-755
- Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 2016; 19 (01) 82-92
- Hattersley R, Nana M, Lansdown AJ. Endocrine complications of immunotherapies: a review. Clin Med (Lond) 2021; 21 (02) e212-e222
- Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 2014; 21 (02) 371-381
- de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res 2019; 51 (03) 145-156
- Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 2019; 40 (01) 17-65
- Barroso-Sousa R, Barry WT, Garrido-Castro AC. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4 (02) 173-182
- Higham CE, Olsson-Brown A, Carroll P. et al; Society for Endocrinology Clinical Committee. Society for Endocrinology Endocrine Emergency Guidance: acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect 2018; 7 (07) G1-G7
- Vaidya B, Chakera AJ, Dick C. Addison's disease. BMJ 2009; 339: b2385
- Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs 2019; 6 (02) 154-160
- Roth P, Winklhofer S, Müller AMS. et al. Neurological complications of cancer immunotherapy. Cancer Treat Rev 2021; 97: 102189
- Cuzzubbo S, Javeri F, Tissier M. et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017; 73: 1-8
- Neelapu SS, Tummala S, Kebriaei P. et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15 (01) 47-62
- Gust J, Hay KA, Hanafi LA. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017; 7 (12) 1404-1419
- Santomasso BD, Park JH, Salloum D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 2018; 8 (08) 958-971
- Xu Y, Li S, Wang Y. et al. Induced CD20 expression on B-cell malignant cells heightened the cytotoxic activity of chimeric antigen receptor engineered T cells. Hum Gene Ther 2019; 30 (04) 497-510
- Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 2018; 32 (12) 1091-1101
- Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2021; 17: 1-12
- Lee DW, Gardner R, Porter DL. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124 (02) 188-195
- Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med 2018; 66 (02) 50-52
- Neelapu SS, Locke FL, Bartlett NL. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377 (26) 2531-2544
- Nastoupil LJ, Jain MD, Feng L. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol 2020; 38 (27) 3119-3128
- Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 2015; 20 (07) 812-822
- Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 2015; 6 (06) 3479-3492
- Puzanov I, Diab A, Abdallah K. et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017; 5 (01) 95
- https://www.onclive.com/view/providing-nursing-insight-into-the-management-of-iraes-in-lung-cancer Accessed December 21, 2021
- Naing A, Hajjar J, Gulley JL. et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer 2020; 8 (02) e001754


 PDF
PDF  Views
Views  Share
Share

