Impacts of a biobank: Bridging the gap in translational cancer medicine
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(01): 17-23
DOI: DOI: 10.4103/0971-5851.151773
Abstract
The prevalence of people affected by cancer has been steadily increasing. More and more people are being offered the chance of increased longevity. This has been possible due to advances not only in medicines and techniques but also because of the gain in understanding of cancer biology through Translational Cancer Medicine. A significant step towards obtaining this success was the establishment of successful biobanking practise. In this review we discuss about the importance of a Biobank and the various impacts that a biobank can have not only in the field of cancer but also on many other aspects. Later we discuss a method of quantitative evaluation of these impacts of a biobank.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The prevalence of people affected by cancer has been steadily increasing. More and more people are being offered the chance of increased longevity. This has been possible due to advances not only in medicines and techniques but also because of the gain in understanding of cancer biology through Translational Cancer Medicine. A significant step towards obtaining this success was the establishment of successful biobanking practise. In this review we discuss about the importance of a Biobank and the various impacts that a biobank can have not only in the field of cancer but also on many other aspects. Later we discuss a method of quantitative evaluation of these impacts of a biobank.
INTRODUCTION
As per the estimate of International Agency for Research in Cancer, in India 948 858 new cases of cancer were detected, and 635,000 people died from cancer in 2008, accounting for 8% of global cancer deaths and 6% of all deaths in India.[1] Due to the increased longevity and increasing control over various communicable and noncommunicable diseases, cancer is set to become one of the most common causes of death despite the dramatic progress in detection and treatment options. It has been estimated that the total cancer cases are likely to go up to 1,148,757 cases in the year 2020.[2]
Translational research is aptly defined as “the effective translation of the new knowledge, mechanisms, and techniques, generated by advances in basic science research, into new approaches for prevention, diagnosis, and treatment of diseases” by Fontanarosa and DeAngelis[3] Commonly referred to as the “bench to bedside” translation of knowledge from basic sciences to produce betterment of clinical practice, translational research is especially applicable in the field of cancer research. The first aspect of translational research involves the transfer of new understandings of disease mechanisms gained in the laboratories into the development of new molecules, methods or devices suitable for human use. The second part involves testing the molecule in human subjects and translation of results from these studies into standard of care clinical practice and health decision making.[4] Furthermore, reverse translation research is described as taking the understanding gained by observations in a patient population to formulate hypotheses and confirmation of the hypothesis in laboratories working on cancer cell lines or animal models. Reverse translational research gives an understanding of the mechanisms of clinical observations and practice.
Central to this exciting field of translational cancer research is the need for a sustainable supply of well-documented and high quality human tissue samples. In vitro and in vivo model systems give scientists an understanding of the molecular deregulation underlying the cancer phenotype, but without evaluation and validation of the same pathways in human tissue the importance of such research would be undermined.[5] Human bio-specimen resources like biobanks are the foundation of basic and translational research and are fundamentally essential to achieve the target of “personalized medicine.”[6]
The goal of this review article is to explain what a biobank is, the various types of existing biobanking and then to illustrate the impacts of a biobank in the field of translational cancer research. The following impacts of a biobank will be included in the discussion
- Impact on clinical research
- Impact of the biobank economic drivers - Physical and Human capital, bioinformatics and standard operating procedures(SOPs)
- Reduced research costs
- Improvement in patient care and treatment cost
- Study of evolution and genetic heterogeneity of cancer.
At the end of this review, an objective method of evaluating the impact of a bio-resource, like a biobank, would be discussed.
WHAT IS A BIOBANK?
A biobank is defined as a collection of bio-materials and relevant clinical-pathological, epidemiological and/or bio-molecular data. The fields of basic, epidemiological and translational research, whether from within or across different centers, find convergence, interoperability and integration on the critical platform of a biobank.[7] Figure 1 shows these multidisciplinary partners and processes required for the successful functioning of a biobank.
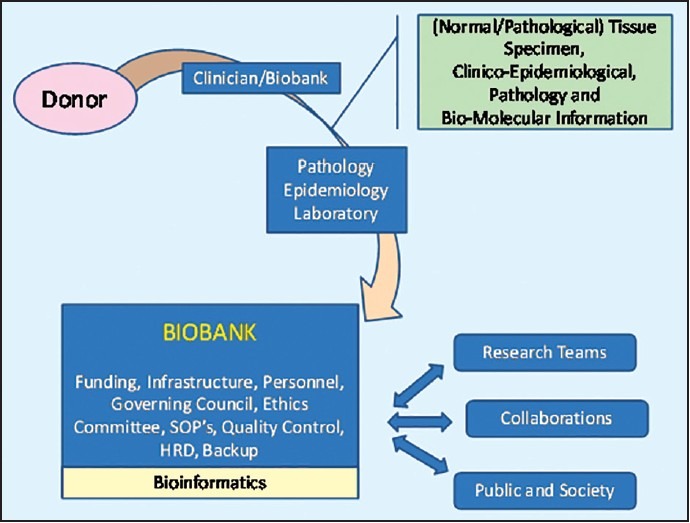
| Fig. 1 Partners and processes of a biobank
A biobank thus collects and stores tissue using validated SOPs. In addition, clinico-epidemiological information linked to the collected tissue samples is captured so that independent research teams can approach the biobank for acquisition of bio-material in relation to translational medical research.
The tissue can be accumulated either associated with a clinical trial or independently for future clinical trials. An additional collection of specimen as part of the trial could be advantageous as these patients are closely followed anyway, so most clinical information related to the tissue is already collected.[8] Biobanking can be in the context of being disease-specific or may be population-based. Disease-specific biobanks collect pathological tissue samples and information from patients suffering from a specific disease, for example, breast cancer or prostate cancer. Disease-specific biobanks have a greater impact on the research on discovery of bio-markers, targeted drug development and in-general, research on treatment of diseases or cancers.[9,10] Population-based biobanks collect normal tissue samples from volunteers and prospectively follow-up for health related parameters and future occurrence of common and complex diseases to primarily study the associative relationships of different environmental or genetic factors for diseases in large populations.[9,11]
The availability of these large collections of well-documented, up-to-date epidemiological, clinical, and biological information, and corresponding tissue specimen is essential for translational research.[12] Some specific examples of the type of research studies mandating such an effort are
- Linkage studies research to identify the genes or genome regions or DNA sequence anomalies as causative for specific disorders like familial syndromes.
- Association studies of diseases use single nucleotide polymorphisms and help identify differences in genetic allele frequency between individuals affected by diseases and controls.[13]
- Prognostic and predictive biomarker discovery using cell surface or intracellular protein detection and associations, using techniques of immunohistochemistry or molecular pathology. Immunological circulating bio-markers would require simultaneous collection of blood or serum.
- Population-based epidemiologic studies to unravel the interactions between genetics, incidence and characteristics, a natural course and the response to treatment.[14]
- Translational research on cell signaling pathways and identification of drug targets and pharmacologic drug discoveries.
- Pharmacogenetic studies relate to understanding the metabolism of the drug in human beings. Identification of sensitivity to certain drugs on the basis of genetic heterogeneity or susceptibility to certain adverse events will lead to further personalization of medicine.[15]
The necessity of these significantly large numbers of bio-specimen required, led to national and international efforts for centralization of the programs and guidelines: GenomEUtwin (www.genomeutwin.org), EuroBioBank (European Network of DNA, cells and tissue banks for Rare Diseases, www.eurobiobank.org), NUGENOB (Nutrient-Gene Interactions in Human Obesity, www.nugene-nob.com), PHOEBE (Promoting Harmonization of Epidemiological Biobanks in Europe, www.phoebe-eu.org), and BBMRI (Biobanking and Bio-molecular Resources Research Infrastructure, www.bbmri.eu) are few such efforts.[16]
These collaborations function mostly in one of the two functioning strategies. The first of them is the “centralized model” where the bio-specimen samples are physically collected at a central biobanking facility from all peripheral collection sites. It is the central nodal biobank which stores, processes and maintains the bio-specimen. Research groups can gain access to the tissue and correspond information only through the central biobank. The second functioning strategy is the “virtual biobank” or a “federated model” in which the samples remain at the peripheral collection sites, but the collections are combined in a virtual sense by transferring all information regarding the tissue sample, to a central database.
IMPACTS OF BIO-BANK
As early as in the 1960's the classical “Framingham Heart Study” showed that people with high cholesterol levels were more prone to develop heart disease. Using this clinical observation, the causative lipoprotein fractions were identified in the laboratories and specific drugs were developed. Currently, the use of these drugs has decreased the risk of heart disease by 30% in this population.[17,18,19,20,21,22] The Framingham heart study has now been re-designed incorporating cutting-edge science embracing elements of basic, translational and population research.[23] Such practical demonstration of the usefulness of tissue storage and information networks from the biobanks can help better the understanding of sciences today. Now let us understand the impacts that a biobank creates, in certain aspects of translational research, in details.
IMPACT ON CLINICAL RESEARCH
The translational impact of a biobank can be looked at purely from the qualitative contribution to science and research. There is plenty of prospectively studied literature available reporting the opportunities promised by biological studies of biomedical research functions.[24] Such an establishment leads to the collection of a broad range of tumor and normal tissue, especially of rare diseases, thereby giving access to the research teams to good quality samples. A biobank serves as a center of excellence for best practice and training, by being a valuable educational resource. Research teams undertaking specific population or disease-specific studies get guidance and access to information from biobanks regarding collection and preservation of tissue locally. Having established leading standards in pathology and being a source of standardized human tissue samples, a biobank becomes a leader in validation of other such establishments.
A biobank provides a sustainable supply of good quality bio-specimens to researchers, thereby reducing the experimental bias and adding to the value of clinical research results. The outcome of such studies are more predictive and give more opportunities for personalization of medicine.[15] Finally, the extensive network established by a biobank, comprising of government and public stakeholders, helps bring together scientists, clinicians, academic researchers, the pharmaceutical industry and patient advocacy research groups, resulting into a stronger collaborative milieu in the scientific community.
The UK biobank project (http://www.ukbiobank.ac.uk/) is a good example of such an endeavor. Over the years 2006-2010, the UK biobank recruited about 500,000 people, aged between 40 and 69 years, from across UK. They have collected blood, urine and saliva samples along with various measures and their detailed health information. The recruited population has also agreed to have regular follow-ups. Established by the Welcome Trust, Medical Research Council, Department of Health, Scottish Government and Northwest Regional Development agency, the UK biobank will be a very valuable long term prospective epidemiological reserve. Already more than 140 project applications from researchers have been submitted for the utilization of this resource.[25]
The North German Tumor Bank of Colorectal Cancer (ColoNet) (http://www.northgermantumorbank-crc.de/North_German_Tumor_Bank_-_CRC/Home.html) is a disease-specific project funded by the German Cancer Aid Foundation involving universities and university clinics in Lübeck, Rostock and Hamburg in Germany. Begun in 2010, the network now stores 6,380 fresh frozen tissue and paraffin embedded samples of colorectal cancers and 7,300 blood samples of healthy individuals and patients with colorectal cancer, metastasis, inflammatory bowel disease and adenomas. Furthermore, ColoNet also generates primary cell cultures and xenograft models.[26] The ColoNet provides the entire network with good quality samples for basic research of colorectal carcinogenesis and for translational research for the improvement of early diagnosis, therapy, surveillance and prognosis. In particular, the team in Lübeck has developed a multiplex-screening chip based on nine serum markers for early colorectal cancer detection. ColoNet provided more than 500 serum samples of healthy controls and colorectal carcinoma patients. Additional 1,000 serum samples of the ColoNet are currently being tested by this prototype chip. The multiplex serum chip was not developed with an incentive to replace colonoscopy but to select patients who will benefit the most from the procedure. It is obvious that such a study would not be possible without the infrastructure as provided by ColoNet.[6]
IMPACT OF THE ECONOMIC DRIVERS OF A BIOBANK- INFRASTRUCTURE AND PERSONNEL, BIOINFORMATICS AND STANDARD OPERATING PROCEDURES
The four main economic drivers of a biobank infrastructure are the infrastructure or the physical capital, the personnel or the human capital, bioinformatics and the SOPs.[13,27] Organizations that manage the capital equipment costs of the physical structure of a substantial biobank store large quantities of high-quality bio-specimens and provide excellent standards of freezer and storage facilities. Smaller organizations, not capable of affording the infrastructure, outsource their bio-specimen at a fraction of the cost.[13] Personnel in a biobank are competently trained, and their proficiency is periodically monitored. Furthermore, strict quality control practices and role specific work allotment leads to lean operations, higher quality products and lower waste rates.[28]
Bioinformatics plays a critical role in determining the scientific accomplishments of a biobank.[29] Comprehensive databases incorporate a variety of bio-specimen related information into one interoperable, integrative and safe system. The field of bioinformatics is upcoming and developing rapidly. Research on new drug discovery, genetic profiling, biomarker validation and development-all require systems built to store and link the bio-specimen's highly annotated initial diagnostic data, the data regarding the diagnostic and therapeutic protocols and the resultant clinical outcomes.[13] By adopting standard biobanking operating procedures, a biobank ensures that only fully quality controlled bio-specimens and data are provided to the research community, thereby ensuring maximum possibility of success in proposed research.[30]
The Estonian Genome Centre, University of Tartu (EGCUT - http://www.geenivaramu.ee/en) is a prime example of the above. Established in 2001, with the intention of creating a large-scale population-based biobank, more than 50,000 participants have been recruited so far, representing more than 5% of the Estonian population. The EGCUT have brought together a biobank infrastructure, a technology core laboratory for sequencing and genotyping and a biostatistics and bioinformatics group par excellence. This infrastructure and the availability of high quality scientific expertise have enabled the EGCUT to join large international research consortia like European Network of Genetic and Genomic Epidemiology, Entrained by the Circardian Clock, BBMRI and Genetic Investigations of Anthropometric Traits. EGCUT, over the years, have generated at least 14 large grants from European Union (EU) Commission. Collaborating with scientists all over EU and United States of America, from over 100 projects, the scientists of EGCUT have co-authored about 117 scientific publications in peer reviewed, high profiled journals.[31]
REDUCED RESEARCH COSTS
One of the potential barriers for bio-markers development is the availability of clinical bio-specimens essential for validation and optimization of new assays.[32] One of the main sources of errors in biomarker discovery and translational research is the variations in collection and prestorage procedures and the specifications of storage capabilities of different biobanks. Availability of a large single source of bio-specimen, procured and preserved with validated SOPs ensures optimal quality for the underlying research. The Confederation of Clinical Trials, under the National Health Scheme (NHS) in UK, and the Strategic Tissue Repository Alliance Through Unified Methodology project, along with their other objectives, are developing SOPs that will harmonize and standardize the biobank practices.[33] As reported by Gartner Inc.[34]“Clinical standards can improve the processes in a single clinical trial that result in an estimated 8-month cycle time reduction in study start up, study conduct, data analysis and reporting, which amounts to a £ 8 million in cost savings.”
The UK Cancer research study portfolio currently lists 4,592 recruiting clinical trials in UK of which 625(~14%) are clinical trials addressing various aspects of cancer research.[35] On an average, around £100( 10000) to £800 (
10000) to £800 ( 80000) million are spent by the pharmaceutical company to develop drug candidates. The pharmaceutical industry spent £3.2(
80000) million are spent by the pharmaceutical company to develop drug candidates. The pharmaceutical industry spent £3.2( 315) billion on Research & Development (R&D) in the UK, in the single year of 2003 and 38% of these clinical trials were phase 1 or biological studies according to the McKinsey Report.[36] Various reports have attributed from 25% to 75% of these studies are either delayed or fail due to nonavailability of timely and good quality bio-specimen.[13] The gap is bridged by a biobank, by providing the benefits of dissemination of standardized human tissues and associated best practices for the use in early phase clinical trials, thereby preventing huge financial losses. Also, by increasing knowledge sharing, biobanks provide means to prevent duplication of similar studies and thus the expenses. This provides significant economic savings to the national health services and to the research community.
315) billion on Research & Development (R&D) in the UK, in the single year of 2003 and 38% of these clinical trials were phase 1 or biological studies according to the McKinsey Report.[36] Various reports have attributed from 25% to 75% of these studies are either delayed or fail due to nonavailability of timely and good quality bio-specimen.[13] The gap is bridged by a biobank, by providing the benefits of dissemination of standardized human tissues and associated best practices for the use in early phase clinical trials, thereby preventing huge financial losses. Also, by increasing knowledge sharing, biobanks provide means to prevent duplication of similar studies and thus the expenses. This provides significant economic savings to the national health services and to the research community.
IMPROVEMENT IN PATIENT CARE AND TREATMENT COST
The power of bio-assays, as defined by the sensitivity and specificity, performed on a human bio-specimen is dependent on the molecular and architectural integrity of the bio-specimen.[37] With the implementation of evidence-based biobanking standards, procedures and quality control, biobanks ensure bio-specimen integrity and prevent degradation. This helps improve the quality of translational research which will lead to an impetus in the research biomarker development.[13] Studies on targeted therapies in human cancers have been significantly dependent on identification, validation and use of such bio-markers. The cost benefit to the patients by discovery of specific targets and drug discovery can be clearly shown by the example of Imatinib Mesylate – a targeted drug discovery based on observation of specific translocation in patients with Chronic Myeloid Leukemia. A cost benefits analysis of imatinib showed that such personalized medicines could generate patient cost savings of over $ 40,000 ( 2495200) per each life-year extended.[38] The costs associated with patient's therapeutic care can be significantly reduced by more efficient and rapid detections and treatment of diseases in early stages.
2495200) per each life-year extended.[38] The costs associated with patient's therapeutic care can be significantly reduced by more efficient and rapid detections and treatment of diseases in early stages.
Rogers et al. illustrate this point eloquently by the hypothetical example of how the biobanks could lead to significant financial impacts.[13] They postulate that if faster diagnosis and earlier treatment, by advances due to research of a biobank, would reduce the annual life-year costs of only 2000 patients of just one type of cancer by only $5000, this in itself would generate an annual savings of $ 10 million of the health care costs. Further if we consider the estimated value of a human life-year as $ 50,000 (The international standard amount used by most government and private run health insurance firms),[39] if the improvement of bio-specimen integrity resulted in more accurate diagnosis and better therapeutic management and increased a patient's average life expectancy by just 0.5%, it would mean an increase in human life-year value of $ 19,000 per patient or almost $ 38 million/year for a cohort of as few as 2000 patients.[13] This creates a huge impact on National economic budgets, especially in countries like UK, as most of the population is covered under NHS.
BIOBANKS: AN INSTRUMENT TO STUDY EVOLUTION AND GENETIC HETEROGENEITY OF CANCER
Evolutionary biology, studying the nature of genetic diversity prevailing in the genomes of individuals, generations, populations and society, sheds light on the structure of its biological basis and evolution itself. The Human Genome Project successfully sequenced the whole human genome and discovered some interesting facts. The 2.91 billion base pairs sequence of the euchoromatic portion of the human genome translates for only about 25000 protein coding genes.[40] Only 1.1% of the genome is populated by exons, whereas introns span about 24%, and rest of the 75% of the genome is just intergeric DNA.[40,41] These majority portions of our genome are the persistent tandem repeats, frequently inactivated by random mutations. Though these fractions, still persist as inert remnants in our genome they may give us valuable information on our origin, our lineage and evolution.[15] Futuristic scientific efforts allow us to correlate the dynamics of the genome with its functional and evolutionary contexts. Biobanking on an international scale gets data from populations around the world and provides a wealth of information on linkages and differences between populations and ethnicities. Specifically in the field of cancer, due to the varying risk factors, incidences and gene-environment interactions, between various populations and regions, are an added value for research, making comparative geo - pathological approaches possible, there by accelerating the understanding of genetic impact and heterogeneity of cancer.[7] To evaluate such complexity in the diverse world populations and our species, world-wide collaborative efforts are ongoing. This calls for a cross-cultural consensus with agreements on not only specimen sharing but perhaps more importantly, agreements on sharing intellectual property, copyrights and licenses. As mentioned at the beginning of this essay, such efforts already exist and will lead the translational research field in the future.[13,16] The International HapMap Project (http://hapmap.ncbi.nlm.nih.gov/thehapmap.html.en) is one such multi-national effort to identify and catalogue, genetic similarities and differences in human beings. Genes are affecting health, various diseases, responses to treatment and the environmental factors that affect the occurrence and outcome can be studied using this information. The project is collaboration between scientists and funding agencies from Japan, UK, Canada, China, Nigeria, and the United States of America.
Thus, a biobank is not only a store of bio-specimen but serves many more important purposes. A need was felt to acknowledge bio-resources for the significant contribution they make to the field of translational research. An attempt to objectively quantify the impact and research output of biobanks, the Bio-resource Research Impact Factor (BRIF) has been created. This score is a numerical form of a bio-resource identifier developed to mandate the end-users to recognize, analyze, compare and acknowledge the provenance of these resources.[42] Any such attempt to quantify the impact and quality of a biobank requires consideration of multiple factors as shown in Figure 2.
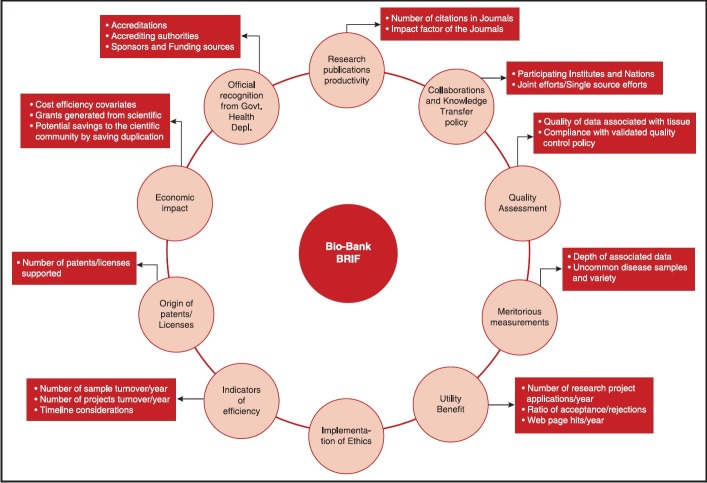
| Fig. 2 Parameters to take into consideration when evaluating the bio-Resource Research Impact Factor of a bio-resource like a biobank - adapted from Cambon-Thomsenet al.[42]
The main objective of a BRIF is to promote the sharing of bio-resources - an underlined need according to a recent editorial in nature medicine.[2] An international working group comprising of 105 participants has been set up under the leadership of Anne Cambon-Thomsen and formulates tools to follow-up the research use of biobanks in the landscape of long term funding. The work involves multiple steps like creating a unique identifier, standardizing bio-resource acknowledgement in scientific papers, cataloguing bio-resource data access and sharing policies, identifying other parameters to take into account and prototype testing, which would involve volunteer bio-resources and help of journal editors.[43] As mentioned previously, policies and agreements of bio-specimen sharing also require policies of intellectual property sharing.
CONCLUSION
Biobanking forms an integral part of Translational Cancer Medicine. Recognizing the importance of a biobank is an essential step to understand the scope of translational research. Apart from providing easy access to large quantities of standardized bio-specimens linked with comprehensive clinical information, biobanks, as illustrated in this essay, can have wider implications. The health care economics, level and cost of patient care, personalized medicine, and even cross-cultural collaborations can be affected either directly or indirectly by a biobank. Understanding and acknowledging the significance of the impacts of biobanks on clinical research needs to be defined and implemented in the future.
ACKNOWLEDGMENT
This review article is based on the submission for the degree of Master of Research (MRes) in Translational Cancer Medicine for King's College London by the first and corresponding author.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Partners and processes of a biobank

| Fig. 2 Parameters to take into consideration when evaluating the bio-Resource Research Impact Factor of a bio-resource like a biobank - adapted from Cambon-Thomsenet al.[42]


 PDF
PDF  Views
Views  Share
Share

