Incidence of Neuropathy with Weekly Paclitaxel and Role of Oral Glutamine Supplementation for Prevention of Paclitaxel Induced Peripheral Neuropathy Randomized Controlled Trial
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(03): 339-348
DOI: DOI: 10.4103/ijmpo.ijmpo_38_17
Abstract
Background: Peripheral neuropathy is damage to the peripheral nerve. The most common cause of neuropathy is paclitaxel. Several avenues have been explored to ameliorate the neurotoxicity associated with paclitaxel. Clinical studies have assessed the efficacy of glutamine with different doses and schedules to prevent gastrointestinal toxicity (mucositis, diarrhea) and peripheral neuropathy in patients receiving a variety of chemotherapy agents or radiation therapy and found that glutamine can prevent paclitaxel-induced peripheral neuropathy. Methods: Total of 50 patients, aged 30 or more with diagnosis of cancer and fulfilling the inclusion and exclusion criteria, formed the study population. We assigned 25 patients to the glutamine group and 25 patients to no glutamine group. All patients received weekly paclitaxel. Results:: The incidence of neuropathy of all grades at 3 months was 78% and at 6 months was 80%.In this study, most common symptom reported was numbness in toes (74%). In this study, Grade 1 was the most common grade of symptom reported by the patient (40%–50%). 2nd, 3rd, and 4th most common grade of symptom reported by the patient was Grade 0, Grade 2, and Grade 3, respectively. There was no Grade 4 symptom reported by any patient. All the symptoms were statistically comparable in both groups (Myalgias: P = 0.066, Arthralgia: P = 0.93, Dysesthesia: P = 0.82, Paresthesia: P = 0.92, Numbness fingers: P = 0.97, Numbness toes: P = 0.60). In our study, there was no incidence of cranial nerve weakness or any incidence of the postural drop. The electrophysiological study is the best tool available and can detect neuropathy at the very earlier stage even when the clinical exam is negative. Apart from that nature of neuropathy can be determined but grading is not possible which makes very difficult to decide on follow-up examinations when the physician should intervene. Moreover, there are fluctuations in SNAP and CMAP, and these fluctuations are most probably related to the innate variability of serial nerve conduction study parameters, particularly motor and sensory amplitude. Glutamine did not prevent neurotoxicity induced by weekly paclitaxel.
Keywords
Glutamine supplementation for prevention of paclitaxel induced neuropathy - Incidence of neuropathy with weekly paclitaxel - Paclitaxel induced neuropathyPublication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Peripheral neuropathy is damage to the peripheral nerve. The most common cause of neuropathy is paclitaxel. Several avenues have been explored to ameliorate the neurotoxicity associated with paclitaxel. Clinical studies have assessed the efficacy of glutamine with different doses and schedules to prevent gastrointestinal toxicity (mucositis, diarrhea) and peripheral neuropathy in patients receiving a variety of chemotherapy agents or radiation therapy and found that glutamine can prevent paclitaxel-induced peripheral neuropathy. Methods: Total of 50 patients, aged 30 or more with diagnosis of cancer and fulfilling the inclusion and exclusion criteria, formed the study population. We assigned 25 patients to the glutamine group and 25 patients to no glutamine group. All patients received weekly paclitaxel. Results:: The incidence of neuropathy of all grades at 3 months was 78% and at 6 months was 80%.In this study, most common symptom reported was numbness in toes (74%). In this study, Grade 1 was the most common grade of symptom reported by the patient (40%–50%). 2nd, 3rd, and 4th most common grade of symptom reported by the patient was Grade 0, Grade 2, and Grade 3, respectively. There was no Grade 4 symptom reported by any patient. All the symptoms were statistically comparable in both groups (Myalgias: P = 0.066, Arthralgia: P = 0.93, Dysesthesia: P = 0.82, Paresthesia: P = 0.92, Numbness fingers: P = 0.97, Numbness toes: P = 0.60). In our study, there was no incidence of cranial nerve weakness or any incidence of the postural drop. The electrophysiological study is the best tool available and can detect neuropathy at the very earlier stage even when the clinical exam is negative. Apart from that nature of neuropathy can be determined but grading is not possible which makes very difficult to decide on follow-up examinations when the physician should intervene. Moreover, there are fluctuations in SNAP and CMAP, and these fluctuations are most probably related to the innate variability of serial nerve conduction study parameters, particularly motor and sensory amplitude. Glutamine did not prevent neurotoxicity induced by weekly paclitaxel.
Keywords
Glutamine supplementation for prevention of paclitaxel induced neuropathy - Incidence of neuropathy with weekly paclitaxel - Paclitaxel induced neuropathyIntroduction
Peripheral neuropathy is damage to the peripheral nerve. Chemotherapy-induced peripheral neuropathy (CIPN) can be due to treatment with multiple chemotherapeutic agents, including platinum compounds (cisplatin, carboplatin, and oxaliplatin), vinca alkaloids (vincristine, vinblastine), taxanes (paclitaxel, docetaxel), thalidomide, and bortezomib. Peripheral neuropathy is a common nonhematological side effect of these drugs. The mechanisms of this neuropathy are usually attributed to microtubule disruption (taxanes, Vinca alkaloids) or a direct toxic effect platinum compounds.[1] The most common cause of neuropathy is paclitaxel. It is dose and infusion duration related. Neuropathy risk is more in patients who have other comorbidities (such as diabetes or kidney disease) or who have been previously treated with other neurotoxic chemotherapy such as cisplatin and vincristine [2],[3]
Neurotoxicity to both small-sensory and large-sensory fibers is seen.[4] It is a sensory predominant neuropathy. Motor neuropathy has been observed with higher doses of paclitaxel.[5] Paclitaxel causes more neuropathy when infused over 3 h as compared to 24 h infusion.[6],[7] Moreover, dose-dense schedule of weekly paclitaxel causes more neuropathy than 3 weekly. Incidence is about 75%. The neuropathy with weekly paclitaxel is generally a sensory polyneuropathy affecting large fibers, can also lead to cranial nerve palsies, motor weakness, and autonomic dysfunction.[8] The options of stopping treatment early or dose reducing are equally undesirable in the advanced disease setting, but may have greater implications in the adjuvant setting because taxanes have become part of the standard treatment for a wide variety of neoplasm, including breast, ovary, lung, and gastrointestinal tumors, first line as well as subsequent therapy.
Several avenues have been explored to ameliorate the neurotoxicity associated with paclitaxel, including the use of nonsteroidal anti-inflammatory agents, corticosteroids, and amifostine and these treatments have been uniformly unsuccessful.[9] Savarese et al.[10] reported the successful reduction of paclitaxel-associated myalgias and arthralgias by glutamine in five patients treated with paclitaxel doses ranging from 175 to 200 mg/m 2. All of the patients had debilitating paclitaxel-associated myalgias/arthralgias associated with their first cycle of therapy. For subsequent cycles, they received glutamine (10 g p. o. t. i. d.) 3 for 4 days starting 24 h after the completion of paclitaxel. No patient had a recrudescence of symptoms while on glutamine.
Glutamine is a neutral gluconeogenic nonessential amino acid stored primarily in skeletal muscle (75%) and liver (25%). Among its many functions, glutamine serves as the primary carrier of nitrogen between tissues. It is also the main energy source for rapidly proliferating cells such as intestinal epithelium, activated lymphocytes, and fibroblasts. Glutamine is depleted in stress states such as major surgery, sepsis, and cancer.[11] It is also essential for maintenance of gut epithelium for patients on total parenteral nutrition as its omission hastens villous atrophy.[12] Preclinical data suggest that glutamine supplementation does not augment tumor cell growth and may augment response to chemotherapy.
Clinical studies have assessed the efficacy of glutamine with different doses and schedules to prevent gastrointestinal toxicity (mucositis, diarrhea) and peripheral neuropathy in patients receiving a variety of chemotherapy agents or radiation therapy and found that glutamine can prevent paclitaxel-induced peripheral neuropathy. Most robust data on prevention of paclitaxel-induced neuropathy is the study done by Stubblefield et al.[13] However, there are no studies on prevention of paclitaxel in India and no randomized trial has been done to look for the incidence of neuropathy with weekly paclitaxel as compared to 3 weekly.
The objective of this randomized study was to evaluate the incidence, clinical presentation of paclitaxel-induced neuropathy and whether it can be prevented by glutamine. Further effort was made to see whether electrophysiological studies can be used to quantify paclitaxel-induced neuropathy.
Methods and Materials
From April 2013 to November 2014, a total of 50 patients with histologically confirmed malignancies treated at B. L Kapur Superciality hospital, NewDelhi, India were enrolled in the study.
Study design
This was randomized controlled trial.
Randomization was done with a total of 50 opaque, sealed envelopes, containing an identifier for glutamine group (Group 1) and containing an identifier for control group (Group 2), shuffled. The order of the shuffled envelopes determined the allocation of participants to treatments.
Patients were randomly assigned to one of the treatment arms.
-
Glutamine group: (Group 1)
-
Control group: (Group 2).
All patients enrolled received weekly paclitaxel as per indications. The dose of paclitaxel was 80 mg/m 2 intravenous over 1 h weekly.
All patients enrolled in glutamine group (Group 1) received oral glutamine 15 g daily (that is approximately 0.25 mg/kg) for 6 months. Glutamine was provided free of cost to all the patients for the entire duration of treatment.
Moreover, patients from the CONTROL group (Group 2) did not receive glutamine or any kind of supplementation apart from routine medications [Figure 1].
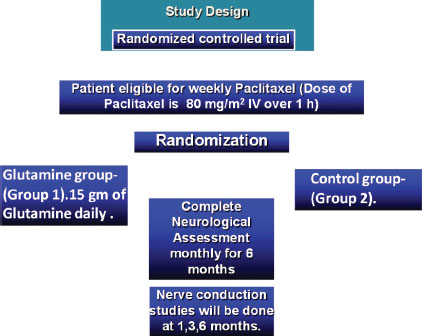
| Figure 1:Study design
Sample size
A total of 50 patients were included (25 patients for each group).
Sample size calculation was based on the study by Linda Vehdat et al.[27] “Reduction of Paclitaxel-induced Peripheral Neuropathy with Glutamine” that showed numbness in toes was reduced by 40% in glutamine arm (intervention arm). Thus, assuming minimum 40% change is clinically relevant to show the effectiveness of the intervention, a sample size of total of 50 patients and 25 patients in each group was calculated. All calculation was done by assuming an error of α =5% (probability of type I error) to achieve β =80% power of the study. Calculation based on this formula: n = f (α/2, β) × (p1× [100 − p1] + p2× [100 − p2])/(p2 − p1) 2.
Inclusion criteria
-
Patients eligible for paclitaxel therapy as per indications
-
Performance status (ECOG)-0–2
-
Age >18 and <80>
-
Patients with established malignancies by histopathological examination report
-
Written informed consent.
Exclusion criteria
-
Central nervous system metastases
-
Prior treatment with taxanes
-
Severe hypersensitivity to taxanes
-
Compromised organ function
-
Baseline neuropathy
-
Life expectancy <6>
-
Abnormal hematopoietic function. Total leukocyte count <3000>
-
Pregnant and lactating mothers.
Written informed bilingual consent was taken from all the subjects. This study has been approved by the ethical committee (clinical) and scientific committee of Dr. B. L. Kapur Superciality Hospital.
Baseline complete blood investigations were done including complete hemogram, liver function tests, kidney function tests to see fitness for chemotherapy. Vitamin B-12 and folate levels were also done. If Vitamin B-12 and folate levels below the reference range below the reference range were replaced.
Data collection methods
Data were collected by direct observation, clinical examination, and electrodiagnostic studies.
Data were recorded for each patient using a structured pro forma which included.
Neurologic evaluation
-
The detailed neurological examination was done before the start of paclitaxel and thereafter was done monthly for 6 months
-
A single reference neurologist (CB) examined all patients at baseline and at 1 (median 30 days) after giving paclitaxel
-
Two patients had paired exams conducted by a single neurologist at an outside institution
-
Previous neurological assessments were not blinded to the examiner
-
A detailed neurologic history was obtained, including possible risk factors for the development of peripheral neuropathy (diabetes, alcohol abuse, or prior history of neurotoxic chemotherapy or neuropathy)
-
A peripheral neuropathy assessment instrument was used to facilitate and standardize data collection.
Grading of neuropathy
-
Questions assessing the symptom of myalgia, arthralgia, numbness of toes and fingers, paresthesia, dysesthesia, was queried separately for fingers and toes and was graded according to NCI-CTC revised version 1999 into five grades (Grade 0 to Grade 5). The highest grade reported at anytime during 6 months by the patient was taken for analysis and comparison
-
Grading of neuropathy was done according to NCI-CTC revised version 1999 into 4 grades (Grade 0–4) monthly for 6 months and examination done at 3 and 6 months was taken for analysis and comparison.
Clinical examination of neuropathy
-
Clinical examination including vibration, grading of power in muscles, reflexes, cranial nerve examination, postural drop was done at baseline and monthly for 6 months. Examination findings at baseline, 3 and 6 months were taken for assessment and comparison
-
Following clinical examination variables were taken for assessment:
-
Vibration at toes at baseline, 3 and 6 months
-
Vibration at ankles at baseline, 3 and 6 months
-
Knee reflexes at baseline, 3 and 6 months
-
Power in left and right dorsiflexors at baseline, 3 and 6 months
-
Cranial nerve weakness at baseline, 3 and 6 months
-
Postural drop baseline, 3 and 6 months.
-
Electrophysiological studies
All patients were evaluated by nerve-conduction studies at baseline, 3 and 6 months.
Motor and sensory responses were recorded using standardized equipment and techniques.
Previous electrophysiological assessments were not blinded.
Variables taken for assessment were:
-
Sensory nerve action potential (SNAP) of left and right sural nerve at baseline, 3 and 6 months
-
The distal latency of right and left sural nerve at baseline, 3 and 6 months
-
SNAP of right and left superior peroneal nerve at baseline, 3 and 6 months
-
Compound motor action potential (CMAP) of right and left common peroneal nerve at baseline, 3 and 6 months
-
CMAP of right and left posterior tibial nerve at baseline, 3 and 6 months.
Statistical analysis
Data were analyzed by using MS Excel and SPSS Statistics for Windows, version 15 (SPSS Inc., Chicago, Ill., USA). Quantitative data (age, cycles received, % change in the mean of CMAP, SNAP and distal latencies) was expressed in terms of mean ± standard deviation. Qualitative data (Neuropathy Grading, Symptom Grading-Myalgia, Arthralgia, Paresthesia, Dysesthesia, Numbness of toes and Fingers and Vibration sensation) was expressed as frequency and percentage.
Statistical significant change in quantitative variable was estimated by paired t-test. The proportion of qualitative variable in control and case was tested by Chi-square test. All the statistical tests performed at 5% level of significance.
A value of P < 0>
Results
A total of 50 patients, aged 30 or more with diagnosis of cancer and fulfilling the inclusion and exclusion criteria, formed the study population. Mean age was comparable between the both groups. The age distribution of the patients was included in this study. Most of the patients in this study were elderly aged ≥50 years of age. Among them, 37 were female and 13 were male and were equally distributed between the groups.
Mean of number of chemotherapy cycles in both the groups was same. In glutamine group, chemotherapy was stopped in one patient and in control group chemotherapy was stopped in two patients due to neurotoxity. In glutamine group, Out of 50 patients, three patients had diabetes mellitus (DM) in each group and total patients with comorbidities were 8 out of 50 patients.
Neuropathy was graded as per NCI-CTC Scale and it was observed that 52% of patients developed Grade 1 neuropathy, 18 % developed Grade 2 months and 8 % developed Grade 3 neuropathy at 3 months. At 6 months, there was an increase in Grade 2 neuropathy by 4% and grade by 3 by 2%. Results were comparable between the groups at 3 months (P = 0.949) and 6 months (P = 0.938). There was no Grade 4 neuropathy in either of the groups [Table 1], [Table 2] and [Figure 2], [Figure 3].
|
NCIC-CTC at 3 months after starting paclitaxel |
Total |
P |
||||
|---|---|---|---|---|---|---|
|
Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
|||
|
NCIC-CTC: National Cancer Institute-Common Toxicity scale |
||||||
|
Glutamine, n (%) |
5 (20.00) |
14 (56.00) |
4 (16.00) |
2 (8.00) |
25 (100.00) |
X2=0.356 |
|
Control, n (%) |
6 (24.00) |
12 (48.00) |
5 (20.00) |
2 (8.00) |
25 (100.00) |
P=0.949 |
|
Total, n (%) |
11 (22.00) |
26 (52.00) |
9 (18.00) |
4 (8.00) |
50 (100.00) |
|
|
NCIC-CTC at 6 months after starting paclitaxel |
Total |
P |
||||
|---|---|---|---|---|---|---|
|
Grade |
0 |
1 |
2 |
3 |
||
|
NCIC-CTC: National Cancer Institute-Common Toxicity scale |
||||||
|
Glutamine |
5 (20.00) |
13 (52.00) |
5 (20.00) |
2 (8.00) |
25 (100.00) |
X2=0.458 |
|
Control |
5 (20.00) |
11 (44.00) |
6 (24.00) |
3 (12.00) |
25 (100.00) |
P=0.938 |
|
Total |
10 (20.00) |
24 (48.00) |
11 (22.00) |
5 (10.00) |
50 (100.00) |
|
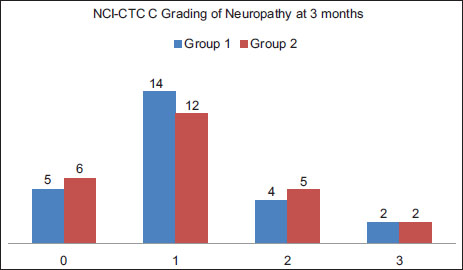
| Figure 2:Paclitaxel-induced neuropathy as per NCI-CTC
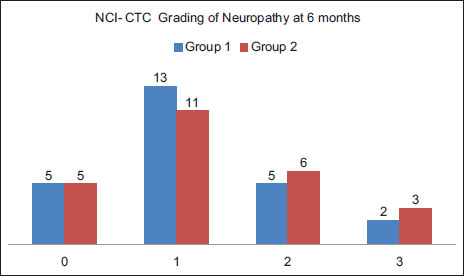
| Figure.3:Grade of neuropathy at 6 months after paclitaxel treatment
The incidence of neuropathy of all grades of neuropathy at 3 months was 78% and at 6 months was 80%.
We graded symptoms of paclitaxel-induced neuropathy (Myalgias, Arthralgias, Paresthesias, Dysesthesias, Numbness of fingers, and Numbness of toes) with NCI-CTC Scale and assessment was done for the highest grade recorded by the patient during treatment for 6 months.
In this study, Grade 1 was the most common grade of symptom reported by the patient (40%–50%). 2nd, 3rd, and 4th most common grade of symptom reported by the patient was Grade 0, Grade 2, and Grade 3, respectively. There was no Grade 4 symptom reported by the patient.
In this study, most common symptom reported was numbness in toes (74%).
All the symptoms were statistically comparable in both groups (Myalgias: P =0.066, Arthralgia: P = 0.93, Dysesthesia: P = 0.82, Paresthesia: P = 0.92, Numbness fingers: P = 0.97, Numbness toes: P = 0.60).
Vibration sense in Ankles was absent in 13 patients (26%) at 6 months and vibration sensation in ankles was absent in 28 (56%).
There was a trend toward less vibration loss in glutamine group, but at follow-up of 6 months, this advantage was lost. Results at 3 and 6 months were statistically comparable in toes as well as in ankles.
Only 1 patient had Grade 4 weakness in dorsiflexors at 3 months in glutamine group that persisted until 6 months. Results were statistically comparable at 3 months (P = 0.31) and 6 months (P = 1).
Lower extremity reflexes were more likely to be preserved in both the groups. Only 5 patients had hyporeflexia at the end of 3 months in both groups which persisted until 6 months. Results were statistically comparable (At 3 months P = 0.69 and 6 months
P = 0.69).
In our study, there was no incidence of cranial nerve weakness or any incidence of the postural drop. One patient had a postural drop at the start of chemotherapy which persisted throughout the treatment and did not worsen during the treatment [Table 3] and [Figure 4].
|
Knee reflexes baseline |
Knee reflexes 3 months |
Knee reflexes 6 months |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Normal |
Hyporeflexia |
Areflexia |
Normal |
Hyporeflexia |
Areflexia |
P |
Normal |
Hyporeflexia |
Areflexia |
P |
|
|
Glutamine |
25 |
0 |
0 |
23 |
2 |
0 |
X2=0.22 |
23 |
2 |
0 |
X2=0.22 |
|
Control |
25 |
0 |
0 |
22 |
3 |
0 |
P=0.69 |
22 |
3 |
0 |
P=0.69 |
|
Total |
50 |
0 |
0 |
45 |
5 |
0 |
45 |
5 |
0 |
||
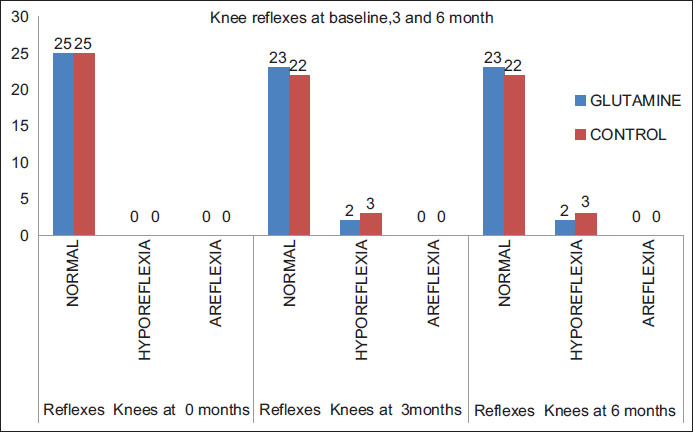
| Figure.4:Knee reflexes at baseline, 3 and 6 months
In our study, the percent changes of the mean SNAP amplitudes of right sural and left sural nerve before and after weekly paclitaxel treatment for both the control group and the glutamine group. The percent changes of the mean distal latency of left sural and right sural nerve before and after weekly paclitaxel treatment for both the control group and the glutamine group.
Percent change of the mean SNAP amplitude was more pronounced in the control group than the glutamine group for both right and left sural nerve at 3 months as well as 6 months but did not reach statistical significance.
Percentage change of the mean distal latency of left sural nerve at 3 months was more pronounced in control group, but at 6 months, it was more pronounced in glutamine group. Percentage change of mean distal latency of right sural nerve at 3 and 6 months was more pronounced in glutamine group at 3 and 6 months as well [Table 4].
|
Glutamine |
Control |
Percentage difference |
P |
|
|---|---|---|---|---|
|
SNAP – Sensory nerve action potential |
||||
|
Left sural nerve SNAP 3 months |
20.66 |
23.82 |
3.16 |
0.51 |
|
Right superior peroneal Nerve 3 months |
10.83 |
28.83 |
5.46 |
0.1 |
|
Right superior peroneal Nerve 6 months |
24.53 |
29.44 |
4.9 |
0.1 |
|
Left superior peroneal nerve 6 months |
19.24 |
16.80 |
2.44 |
0.49 |
|
Left superior peroneal nerve 6 months |
24.91 |
22.67 |
2.24 |
0.54 |
|
Left sural nerve distal latency 3 months |
16.52 |
20.63 |
4.11 |
0.45 |
|
Left sural nerve distal latency 3 months |
26.54 |
22.66 |
3.88 |
0.35 |
|
Right sural nerve distal latency 3 months |
19.64 |
16.49 |
3.15 |
0.37 |
|
Right sural nerve distal latency 3 months |
27.13 |
24.82 |
2.31 |
0.52 |
|
Glutamine |
Control |
Percentage difference |
P |
|
|---|---|---|---|---|
|
SNAP – Sensory nerve action potential |
||||
|
CMAP |
||||
|
Left common peroneal nerve 3 months |
4.46 |
4.38 |
0.14 |
0.98 |
|
Left common peroneal nerve 6 months |
5.52. |
4.95 |
0.57 |
0.81 |
|
Right common peroneal nerve 3 months |
7.9 |
7.11 |
0.87 |
0.78 |
|
Right common peroneal nerve 6 months |
10.43 |
9.83 |
0.6 |
0.88 |
|
Left posterior tibial nerve 3 months |
6.01 |
5.80 |
0.21 |
0.95 |
|
Left posterior tibial nerve 6 months |
7.5 |
6.9 |
0.64 |
0.86 |
|
Right posterior tibial nerve 3 months |
3.34 |
3.13 |
0.23 |
0.83 |
|
Right posterior tibial nerve 6 months |
4.05 |
4.29 |
0.24 |
0.34 |
|
At 3 months |
At 6 months |
||
|---|---|---|---|
|
Grade % |
Total (78%) |
Grade % |
Total (80%) |
|
Grade 0 |
22% |
Grade 0 |
20% |
|
Grade 1 |
52% |
Grade 1 |
48% |
|
Grade 2 |
18% |
Grade 2 |
22% |
|
Grade 3 |
8% |
Grade 3 |
10% |
|
Grade 4 |
0 |
Grade 4 |
0 |
|
Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
P |
|
|---|---|---|---|---|---|---|
|
Myalgia |
||||||
|
Glutamine |
10 |
10 |
4 |
1 |
0 |
0.066 |
|
Control |
4 |
13 |
5 |
3 |
0 |
|
|
Arthralgia |
||||||
|
Glutamine |
3 |
17 |
3 |
2 |
0 |
0.93 |
|
Control |
11 |
8 |
3 |
3 |
0 |
|
|
Dysesthesia |
||||||
|
Glutamine |
6 |
12 |
5 |
2 |
0 |
0.822 |
|
Control |
8 |
9 |
5 |
3 |
0 |
|
|
Paresthesia |
||||||
|
Glutamine |
7 |
12 |
4 |
2 |
0 |
0.920 |
|
Control |
7 |
10 |
5 |
3 |
0 |
|
|
Numbness fingers |
||||||
|
Glutamine |
7 |
11 |
5 |
2 |
0 |
0.970 |
|
Control |
7 |
10 |
5 |
3 |
0 |
|
|
Numbness toes |
||||||
|
Glutamine |
7 |
12 |
5 |
1 |
0 |
0.607 |
|
Control |
6 |
9 |
7 |
3 |
0 |
|
Conflict of Interest
There are no conflicts of interest.
References
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. et al. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006; 33: 15-49
- Lipton RB, Apfel SC, Dutcher JP, Rosenberg R, Kaplan J, Berger A. et al. Taxol produces a predominantly sensory neuropathy. Neurology 1989; 39: 368-73
- Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 1993; 15: 107-15
- Bitton RJ, Figg WD, Reed E. et al. A preliminary risk-benefit assessment of paclitaxel. Drug Saf 1995; 12: 196-208
- Balmaceda C, Freilich RJ, Seidman AD, Rubin M, DeAngelis LM. Motor neuropathy due to docetaxel and paclitaxel. Neurology 1996; 47: 115-8
- Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME. et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: High-dose versus low-dose and long versus short infusion. J Clin Oncol 1994; 12: 2654-66
- Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH. et al. Randomized trial of 3-hour versus 24-hour infusion of high-dose paclitaxel in patients with metastatic or locally advanced breast cancer: National surgical adjuvant breast and bowel project protocol B-26. J Clin Oncol 1999; 17: 3403-11
- Mielke S, Mross K, Gerds TA, Schmidt A, Wäsch R, Berger DP. et al. Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anticancer Drugs 2003; 14: 785-92
- Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, Rowland KM. et al. The use of Vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support Care Cancer 2011; 19: 1769-77
- Savarese D, Boucher J, Corey B. Glutamine treatment of paclitaxel-induced myalgias and arthralgias. J Clin Oncol 1998; 16: 3918-9
- Kautio AL, Haanpää M, Leminen A, Kautiainen H, Saarto T. et al. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptom. Anticancer Res 2009; 29: 2601-6
- ;Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA. et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 2007; 110: 2110-8
- Stubblefield MD, Vahdat LT, Balmaceda CM, Troxel AB, Hesdorffer CS, Gooch CL. et al. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: A clinical and electrophysiologic study. Clin Oncol (R Coll Radiol) 2005; 17: 271-6
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol 1995; 6: 489-94
- Pace A, Bove L, Aloe A, Nardi M, Pietrangeli A. Paclitaxel neurotoxicity: Clinical and neur18, Princeton NJ: Bristol-Myers Squibb. Taxol Prescribing Information. pathophysiologic study of 23 patients. Ital J Neurol Sci 1997; 18: 73-9
- Taxol Prescribing Information. Princeton NJ: Bristol-Myers Squibb; 2011.
- Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK. et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008; 26: 1642-9
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D. et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009; 374: 1331-8
- Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T. et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008; 358: 1663-71
- Pace A, Nisticò C, Cuppone F, Bria E, Galiè E, Graziano G. et al. Peripheral neurotoxicity of weekly paclitaxel chemotherapy: A schedule or a dose issue?. Clin Breast Cancer 2007; 7: 550-4
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: A new approach in clinical testing. Muscle Nerve 2000; 23: 339-409
- Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T. et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol 2011; 29: 421-7
- Wen F, Zhou Y, Wang W, Hu QC, Liu YT, Zhang PF. et al. Ca/Mg infusions for the prevention of oxaliplatin-related neurotoxicity in patients with colorectal cancer: A meta-analysis. Ann Oncol 2013; 24: 171-8
- Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P. et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 2014; 32: 997-1005
- Chroni E, Argyriou AA, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T. et al. Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. Neurology 2006; 67: 2253-5
- Rao RD, Flynn PJ, Sloan JA, Wong GY, Novotny P, Johnson DB. et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer 2008; 112: 2802-8
- Vahdat L, Papadopoulos K, Lange D, Leuin S, Kaufman E, Donovan D. et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res 2001; 7: 1192-7
- Loven D, Levavi H, Sabach G, Zart R, Andras M, Fishman A. et al. Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. Eur J Cancer Care (Engl) 2009; 18: 78-83
- Wang WS, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS. et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 2007; 12: 312-9
Address for correspondence
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure 1:Study design

| Figure 2:Paclitaxel-induced neuropathy as per NCI-CTC

| Figure.3:Grade of neuropathy at 6 months after paclitaxel treatment

| Figure.4:Knee reflexes at baseline, 3 and 6 months
References
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. et al. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006; 33: 15-49
- Lipton RB, Apfel SC, Dutcher JP, Rosenberg R, Kaplan J, Berger A. et al. Taxol produces a predominantly sensory neuropathy. Neurology 1989; 39: 368-73
- Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 1993; 15: 107-15
- Bitton RJ, Figg WD, Reed E. et al. A preliminary risk-benefit assessment of paclitaxel. Drug Saf 1995; 12: 196-208
- Balmaceda C, Freilich RJ, Seidman AD, Rubin M, DeAngelis LM. Motor neuropathy due to docetaxel and paclitaxel. Neurology 1996; 47: 115-8
- Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME. et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: High-dose versus low-dose and long versus short infusion. J Clin Oncol 1994; 12: 2654-66
- Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH. et al. Randomized trial of 3-hour versus 24-hour infusion of high-dose paclitaxel in patients with metastatic or locally advanced breast cancer: National surgical adjuvant breast and bowel project protocol B-26. J Clin Oncol 1999; 17: 3403-11
- Mielke S, Mross K, Gerds TA, Schmidt A, Wäsch R, Berger DP. et al. Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anticancer Drugs 2003; 14: 785-92
- Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, Rowland KM. et al. The use of Vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support Care Cancer 2011; 19: 1769-77
- Savarese D, Boucher J, Corey B. Glutamine treatment of paclitaxel-induced myalgias and arthralgias. J Clin Oncol 1998; 16: 3918-9
- Kautio AL, Haanpää M, Leminen A, Kautiainen H, Saarto T. et al. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptom. Anticancer Res 2009; 29: 2601-6
- ;Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA. et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 2007; 110: 2110-8
- Stubblefield MD, Vahdat LT, Balmaceda CM, Troxel AB, Hesdorffer CS, Gooch CL. et al. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: A clinical and electrophysiologic study. Clin Oncol (R Coll Radiol) 2005; 17: 271-6
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol 1995; 6: 489-94
- Pace A, Bove L, Aloe A, Nardi M, Pietrangeli A. Paclitaxel neurotoxicity: Clinical and neur18, Princeton NJ: Bristol-Myers Squibb. Taxol Prescribing Information. pathophysiologic study of 23 patients. Ital J Neurol Sci 1997; 18: 73-9
- Taxol Prescribing Information. Princeton NJ: Bristol-Myers Squibb; 2011.
- Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK. et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008; 26: 1642-9
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D. et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009; 374: 1331-8
- Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T. et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008; 358: 1663-71
- Pace A, Nisticò C, Cuppone F, Bria E, Galiè E, Graziano G. et al. Peripheral neurotoxicity of weekly paclitaxel chemotherapy: A schedule or a dose issue?. Clin Breast Cancer 2007; 7: 550-4
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: A new approach in clinical testing. Muscle Nerve 2000; 23: 339-409
- Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T. et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol 2011; 29: 421-7
- Wen F, Zhou Y, Wang W, Hu QC, Liu YT, Zhang PF. et al. Ca/Mg infusions for the prevention of oxaliplatin-related neurotoxicity in patients with colorectal cancer: A meta-analysis. Ann Oncol 2013; 24: 171-8
- Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P. et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 2014; 32: 997-1005
- Chroni E, Argyriou AA, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T. et al. Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. Neurology 2006; 67: 2253-5
- Rao RD, Flynn PJ, Sloan JA, Wong GY, Novotny P, Johnson DB. et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer 2008; 112: 2802-8
- Vahdat L, Papadopoulos K, Lange D, Leuin S, Kaufman E, Donovan D. et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res 2001; 7: 1192-7
- Loven D, Levavi H, Sabach G, Zart R, Andras M, Fishman A. et al. Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. Eur J Cancer Care (Engl) 2009; 18: 78-83
- Wang WS, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS. et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 2007; 12: 312-9


 PDF
PDF  Views
Views  Share
Share

